Food as Medicine 2.0: Personalized Nutrition with Microbiome and AI
The “Food as Medicine” paradigm is shifting toward a personalized approach, where nutrition is tailored to an individual’s unique biology, lifestyle, and health needs. This change is driven by advances in genomics, microbiome science, and artificial intelligence (AI), allowing the creation of customized diets that consider factors like gut microbiome makeup, genetic traits, metabolic responses, and behavioral data. The gut microbiome, a complex ecosystem of trillions of microbes, plays a key role in digestion, nutrient absorption, immune regulation, mood, metabolism, and disease prevention. Each person’s microbiome is different and influences how they respond to specific foods. With AI and machine learning, vast biological data from microbiome sequencing, glucose monitoring, food intake, and wearables can now be analyzed to produce dynamic, personalized dietary advice. The personalized nutrition market is growing quickly, attracting significant venture capital, government funding, and innovation from startups and major food companies. Clinical trials have demonstrated that AI-driven diets can perform better than traditional methods in managing conditions like diabetes, IBS, and obesity, highlighting the potential for microbiome-based, tech-enabled precision nutrition to become a key part of preventive healthcare and consumer wellness.
Food is Medicine: “A New Paradigm”

The idea that “food is medicine” is evolving into a new paradigm where diet is tailored to individual biology and life circumstances. Technological advances in genomics, microbiology, and artificial intelligence now make it possible to design diets and foods customized to each person’s genetic profile, gut microbiome, lifestyle, and health goals. Consumers are becoming increasingly health-conscious and expect food products to deliver both nutrition and wellness benefits. This convergence of science, data, and consumer demand is driving “Food as Medicine 2.0” – a movement in which food companies treat eating as a personalized health intervention.
The Microbiome: Your Inner Ecosystem
What is the Gut Microbiome?
The gut microbiome refers to the vast and dynamic community of microorganisms—primarily bacteria, but also viruses, fungi, and archaea—residing in the gastrointestinal tract. Trillions of these microbes live symbiotically with us, playing vital roles in everything from digestion to brain health. While each person’s microbiome is unique (like a fingerprint), the diversity and balance of species within it are critical to our overall health. Let us unravel why it matters:
Digestion and Nutrient Absorption
- The microbiome helps break down complex carbohydrates, fibers, and polyphenols that the human body cannot digest on its own.
- It synthesizes essential nutrients like vitamins B and K, and aids in the absorption of minerals such as magnesium, calcium, and iron.
Immunity and Inflammation Control
- About 70% of the immune system resides in the gut.
- A healthy microbiome educates the immune system, helping it distinguish between harmful invaders and benign substances.
- It maintains the gut barrier integrity, preventing “leaky gut” and the entry of inflammatory toxins into the bloodstream.
Mood and Brain Function (The Gut–Brain Axis)
- Gut microbes produce and modulate neurotransmitters such as serotonin (90% of which is made in the gut), dopamine, and GABA.
- The vagus nerve connects the gut and brain, transmitting signals that can influence mood, anxiety, and cognition.
- Dysbiosis (microbial imbalance) has been linked to depression, anxiety, and neurodegenerative diseases.
Metabolism and Weight Regulation
- Certain gut bacteria influence how we store fat, regulate blood sugar, and feel hunger or satiety.
- A more diverse microbiome is associated with better insulin sensitivity, lower inflammation, and a reduced risk of obesity and type 2 diabetes.
The Power of Personalization
In recent years, we’ve witnessed a profound shift from “one-size-fits-all” nutrition to individualized diet plans powered by cutting-edge science. Central to this evolution is the understanding that each person’s response to food is unique—shaped by factors like gut microbiome composition, genetic markers, metabolic patterns, and lifestyle.
Why Personalized Nutrition Matters:
Traditional dietary guidelines often fail to account for:
- Inter-individual variability in blood sugar or fat responses to the same food
- How different gut microbes break down nutrients
- The role of inflammation, hormonal signals, and gene–nutrient interactions
This has resulted in poor adherence, varied outcomes, and limited long-term effects.
Now, with the rise of AI, machine learning, and gut microbiome science, personalized nutrition is becoming actionable, measurable, and scalable.
Key Drivers of Personalization
| Data Source | Role in Personalization |
| Gut Microbiome | Identifies how individuals digest and metabolize specific foods |
| Genetics | Predicts nutrient needs, intolerances, or sensitivities |
| Biomarkers | Tracks glucose, lipids, insulin, and inflammatory markers |
| Behavioral Data | Captures sleep, stress, and activity patterns |
AI: The Nutrition Engine
As the field of personalized nutrition develops, artificial intelligence (AI) is becoming essential for providing truly individualized dietary advice. By analyzing extensive and complex biological data—from gut microbiome sequencing and genetic tests to wearable devices and food logs—AI models can detect patterns that human experts simply cannot.
Instead of generalized diet plans, AI delivers dynamic, data-driven recommendations based on how your body uniquely responds to food—improving outcomes for weight management, chronic disease prevention, and even cognitive function.
How AI Powers Personalized Nutrition
| Data Input | AI Output |
| Microbiome sequencing (bacteria, RNA, metabolites) | Predicts digestion efficiency, inflammation risk |
| Continuous glucose monitoring (CGM) | Predicts blood sugar spikes for specific meals |
| Food logs and meal composition | Assigns food scores or generates daily plans |
| Wearables (e.g., Fitbit, WHOOP, Apple Watch) | Integrates sleep, activity, HRV, with nutrition timing |
| Genetic data (nutrigenomics) | Detects nutrient sensitivities, metabolic traits |
| Mood/cognition tracking apps | Suggests foods that support focus or reduce anxiety |
Industry Outlook
Market and Investment Growth of Personalized Nutrition
The personalized nutrition sector is experiencing rapid growth and investment. Our recent analysis suggests that the market will cross USD 18 billion by the end of this year and will reach USD 60 billion by 2034. North America currently holds the largest share (≈43% of 2024 revenues), though the Asia-Pacific is growing fastest. These forecasts encompass not just food products but also digital services and supplements – yet a significant portion is food-related as companies bundle recommendations with edible solutions.
Venture capital is flowing into personalized meal platforms and ingredient innovation. In 2024–25, several high-profile deals highlight this trend: Season Health raised about $43.6 million to expand its diabetes nutrition platform.
Wonder, the AI meal-kit startup, secured $600 million (led by Google Ventures) to build its blood-test-driven food service.
Other companies like ZOE and Viome have raised dozens of millions of dollars over the past years to fund large clinical trials and expand globally. Strategic corporate investments are also common; for instance, PepsiCo’s commitment to an AI personalization lab indicates internal R&D funding, and multinationals often partner with startups to incubate new concepts.
Government Initiatives and Regulation
Public-sector support for nutrition-focused health is on the rise, though policies are still catching up to the technology.
- In the United States, the NIH’s Nutrition for Precision Health program (part of the All of Us Research initiative) exemplifies a significant investment – roughly $170 million – to study how individuals respond differently to diets based on genetics, microbiome, and other biomarkers. On the clinical side, government-funded pilot programs are exploring “food as medicine” in public health. For example, U.S. states use CDC grants to run fruit-and-vegetable prescription programs (PPR) where doctors prescribe healthy foods to at-risk patients.
- The European Union has similarly funded research projects integrating nutrition, microbiome, and AI (e.g., the PROTEIN and Stance4Health projects under Horizon 2020). However, EU food regulation remains strict: while claims like “contains live cultures to support digestive health” are allowed on probiotic yogurts, any health or disease-related claims require rigorous scientific substantiation and EFSA approval. This cautious stance means companies must generate strong evidence before labeling foods with personalized health benefits. Data privacy laws (GDPR) also impose rules on collecting and processing genetic/microbiome data, influencing how personal nutrition services operate in Europe.
- In Asia, countries like Singapore and South Korea are launching national precision nutrition initiatives, pairing genomic and microbiome profiling with dietary recommendations.
Commercial Innovation Hubs
A wide range of companies – from nimble startups to food and beverage giants – are active in this space. Key startups include:

Major food and consumer brands are also entering the field. For instance,

Nestlé, Danone, and other global food companies have invested in microbiome research and health-food startups to position themselves for the personalized nutrition market. Retailers and tech firms are also collaborating: meal-kit companies like Blue Apron (acquired by Wonder) and HelloFresh are exploring AI-driven personalization, and data companies are partnering with food brands to analyze customer nutrition data.
AI & Machine Learning for Microbiome-Driven Nutrition
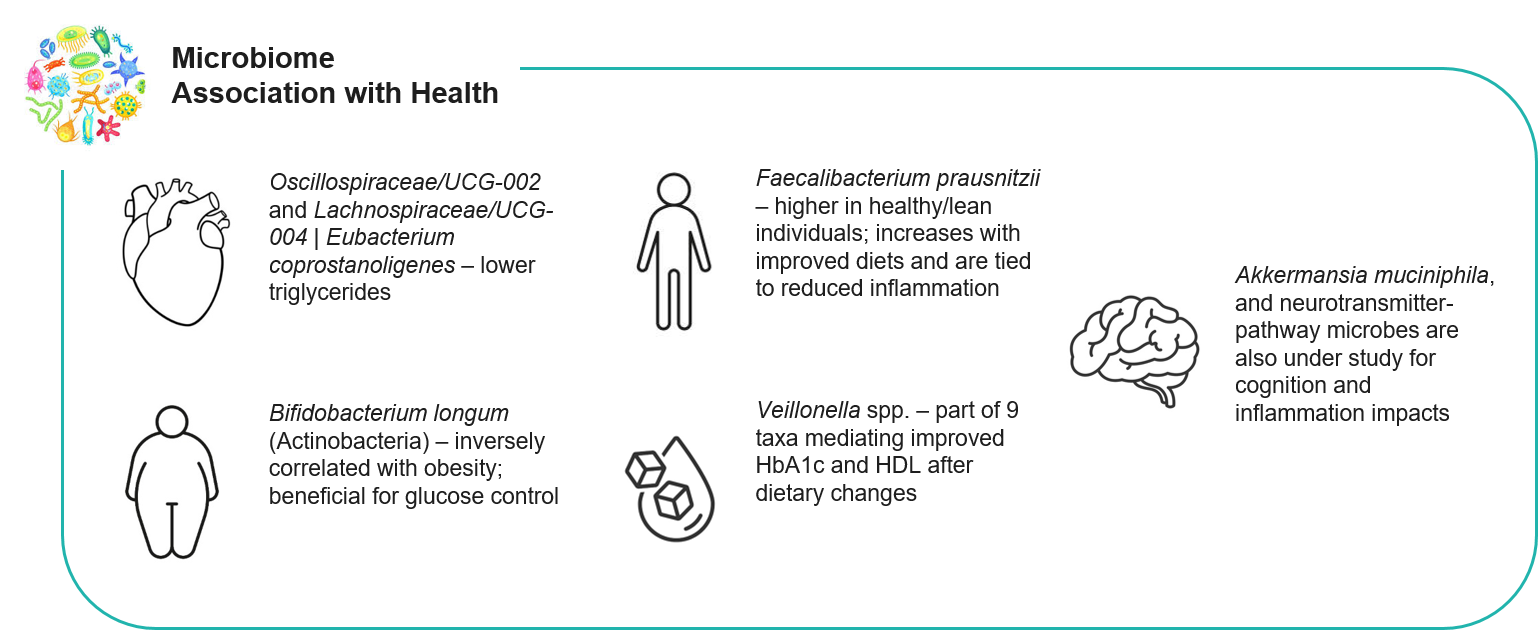
Gut Microbiome Association with Health
Gut microbiota strongly influences metabolism and chronic disease risk, wherein diet both shapes and is modulated by these microbes. Recent years have mapped key gut bacteria linked to obesity, glucose control, inflammation, and even mental health. For example, beneficial SCFA-producers often decline in metabolic syndrome: Faecalibacterium prausnitzii (butyrate producer) tends to be lower in obesity/diabetes, while Bifidobacterium (probiotic genus) generally correlates with leanness. Conversely, higher levels of some Firmicutes (e.g., Eubacterium hallii group) or Proteobacteria (e.g., Veillonella spp.) have been associated with increased BMI and type 2 diabetes risk. In one 2023 dietary intervention study, an AI-guided high-fiber diet raised gut diversity and boosted Oscillobacter and Faecalibacterium (both SCFA-producers) while reducing inflammation-linked Eubacterium ruminantium. Another Mendelian-randomization analysis identified Eubacterium eligens and Veillonella as protective against T2D, whereas Roseburia, Faecalibacterium, Oscillospiraceae, and certain Bacteroides were linked to higher T2D risk. Notably, Bifidobacterium longum and other Actinobacteria were found to be inversely correlated with BMI, suggesting their role in glycemic and weight control. Overall, these and other studies highlight how specific gut microbes (and overall microbiome diversity) underlie individual differences in diet response and chronic disease prevention. Key microbe–health associations:
Integrating Microbiome Data with AI
Integrating microbiome data with AI enables the prediction of individual dietary responses. Deep learning and other ML models can extract complex patterns from high-dimensional gut datasets that traditional analyses miss. For instance, one recent paper describes how a neural network uses a person’s fecal metagenome to predict glycemic and lipid responses to specific foods. In a 200-subject diet trial, an AI model trained on baseline microbiome, diet logs, and clinical data successfully forecasted personalized metabolic outcomes (A1c, HDL, triglycerides) following dietary changes. As one review explains, ML/DL “Adeptly capture the complex interactions within the microbiota” that influence nutrient metabolism.
Using AI models, researchers and apps tailor diets by mapping Food→Microbiome→Metabolism
- Israeli prediabetes trials showed an AI-customized “postprandial-targeting” diet caused larger gut diversity gains and better glucose and lipid outcomes than a standard Mediterranean diet.
- In that study, nine microbial species were identified that partially mediated diet-induced improvements in HbA1c and lipids, underscoring the bidirectional link between diet and microbiome.
- In short, AI learns from prior diet–microbiome–health data to optimize each person’s food choices.
ML Models/Techniques Researchers employ many approaches in microbiome-nutrition studies:

In summary, a variety of AI techniques – from classic ML to cutting-edge DL – are being adapted to learn diet–microbiome–health relationships. These models can predict personalized nutrition responses with increasing accuracy, fueling next-generation diet apps and services.
Clinical Trials and Research Initiatives
Several recent trials and programs specifically target the microbiome–AI nexus in nutrition:
- IBS and Gut Health: A 2022 Turkish RCT (Arslan et al.) randomly assigned functional constipation patients to standard care or an AI-designed diet (made by Enbiosis). The AI diet (high-fiber and microbiome-adjusted) significantly increased complete bowel movements and quality of life vs controls. Similarly, a 2024 multi-center IBS trial in Turkey used patients’ gut profiles to formulate a personalized “Precision Diet” (foods ranked by an ML model); this produced greater IBS symptom relief than a standard low-FODMAP diet.
- Metabolic Disease: An Indian study (Joshi et al., 2023) used a “digital twin” AI system for T2D patients. Over one year, 72.7% of patients on the AI/nutrition program achieved diabetes remission (HbA1c improvement) versus none in control, along with significant reductions in weight, BMI, waist circumference, and liver fat. In Israel, a 6-month RCT (Ben-Yacov et al., 2023) compared a personalized “postprandial” diet (based on an ML glycemic model) to a Mediterranean diet in prediabetics. The ML-driven diet induced bigger improvements in glycemic markers and lipids, correlating with specific microbiome shifts.
- Large Cohort Studies: The UK’s ZOE PREDICT studies tracked >1,000 twins and family members, collecting microbiome, genetics, and continuous glucose data. These analyses confirmed that identical foods elicit highly individual metabolic responses shaped mainly by the gut microbiome (as well as lifestyle and genetics).
- Mobile Apps and Platforms: Academic–industry collaborations (e.g., the Viome Precision Nutrition Program) leverage gut or blood tests plus ML to recommend foods. Early analyses (from user data) suggest that such programs can lower IBS symptom scores, depression/anxiety scores, and even calculate diabetes risk. Other trials embed CGM sensors and wearables to fine-tune AI diet algorithms in real time.
Translation into Food Products and Services
These scientific advances are rapidly spawning real-world applications:


Future Aspect of Personalized Nutrition with Microbiome and AI
The future of Food as Medicine 2.0 depends on the convergence of microbiome science, precision diagnostics, and AI-driven personalization, which will fundamentally change how we understand nutrition and health. As microbiome research advances, we will identify more microbe–health relationships, enabling diets to be tailored with the same precision as drugs. AI models will grow more advanced, combining gut microbiota data with genomic, epigenetic, hormonal, behavioral, and environmental factors to develop highly personalized nutritional plans.
In the next decade, real-time nutrition guidance—driven by wearables, continuous glucose monitors, and gut sensors—may become quite common. Individuals will receive dynamic food recommendations tailored to current stress levels, sleep quality, inflammation markers, or even cognitive load. Hospitals, insurers, and employers will be expected to adopt precision nutrition platforms to reduce the burden of chronic diseases and cut healthcare costs. Simultaneously, the food industry will shift toward personalized product design, with ingredients such as prebiotics, postbiotics, and polyphenols customized to one’s gut profile. As data privacy, accessibility, and regulatory frameworks evolve, microbiome-informed diets may move from early adopters to mainstream medical and consumer use. The result will be a paradigm shift: food will not just prevent illness, but actively enhance energy, cognition, mood, and longevity—ushering in an era where personalized nutrition becomes a core pillar of preventive, predictive, and participatory healthcare.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.