Beyond mRNA: How CircRNA Is Disrupting The Therapeutic Paradigm
Circular RNAs (CircRNA) are a naturally occurring, large family of stable single-stranded RNAs. They are a strongly emerging category in RNA therapeutics and are expected to outgrow their linear counterparts due to their low immunogenicity, higher stability, and stronger protein expression. Early research has scratched only the tip of the iceberg in the last few years. With evolving technologies and support infrastructure, this RNA subclass will reshape the RNA therapeutic world in a whole new way.
Understanding the Basics
CircRNA has been around for decades and was anecdotally mentioned with respect to animals and plants now and then. However, it gained attention with respect to humans around 2010 as a non-coding RNA that was highly conserved. Their unique structural and functional attributes demonstrated a favorable immunological profile and higher stability than mRNAs. In the last decade, the true significance of circRNAs surfaced alongside advancements in circRNA-directed bioinformatics and high-throughput RNA sequencing technologies. The comprehensive understanding of their diversity also ascribes the range of their functions within the cell, underscoring their potential as a therapeutic modality.
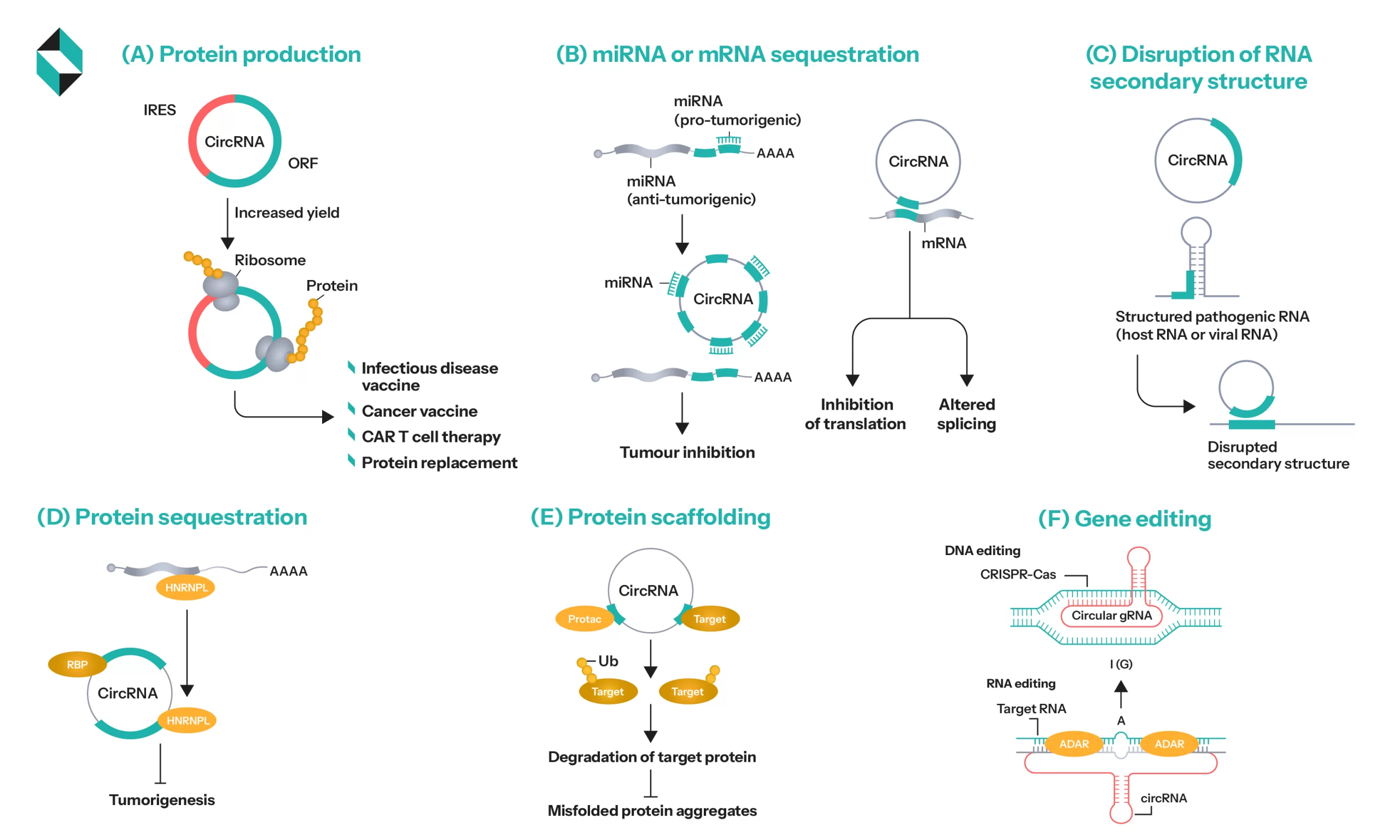
Therapeutic Relevant Functions of circRNA and Its Potential Applications (Source)
Leading Therapeutic Applications
Cancer: CircRNAs are the basis of cancer vaccines and targeted therapeutics. Most circRNA vaccines showcased promising efficiency and superior performance to mRNA vaccines for cancer treatment. For instance, a vaccine called circRNAOVA-luc-LNP successfully elicited an anti-tumor immune response when introduced in a mouse with a tumor. Similarly, in a recent clinical trial, CAR T-cell therapy with sequential mRNA vaccine administration demonstrated strong anti-neoplastic effects in genitourinary cancer patients. There are several other examples where circRNA-based vaccines showcased much higher antigen production than mRNA vaccines in cancer patients. Some even played a key role in drug resistance and slowing down tumor progression, making them a suitable tool for intervention.
Neurodegenerative Diseases: The potential of circRNAs in treating neurodegenerative diseases like Alzheimer’s was recently showcased in collaborative research by Stanford Healthcare and Banner Alzheimer Institute in the USA. It states that the lack of 3’ and 5’ ends in the circular RNA prevents them from being a direct target of endo and exo-nucleases, allowing them to cross the blood-brain barrier and relay therapeutic payload to the needed parts. There is ample evidence that suggests their ability to act as polypeptide-generating templates. It also indicates their crucial role in bone health and critical neurodegenerative disorders like CNS injuries, ischemic strokes, Alzheimer’s, traumatic brain injuries, as well as cancer.
Infectious Diseases: Using this RNA subclass in SARS-COV-2 vaccines proved its ability to combat infectious diseases and yield favorable results. Several research studies are currently investigating the potential application of circRNA in controlling other contagious diseases. However, most of these efforts aim to improve T-cell responses through drugs and vaccines. Further evidence supporting their validity for these problems has yet to surface.
Cardiovascular and Autoimmune Diseases: Several studies are exploring the role of circRNAs in modulating the immune response and optimizing gene pathways involved in these diseases.
Comprehensive Overview of Published circRNA Therapeutics for Various Infectious and Other Diseases (Source)
Challenges
- Efficient Synthesis and circRNA circularization: Current methods, including chemical, ribozyme, and enzymatic processes, struggle to provide higher yields. However, recent advances like Clean-PIE and Group II Intron Systems have helped over 90% of circularization. Scaling these processes, though, is still a question.
- Delivery System Optimization: The tissue specificity and struggles to overcome blood-brain barriers persist with the current delivery strategies, like lipid nanoparticles. Most delivery vehicles trigger unwanted immune responses, complicating the long-term usage of circRNA for various purposes.
- Regulatory Hurdles: The stability, activation of pattern recognition receptors, and insufficient toxicology assessments have slowed regulatory approval for circRNA therapeutics. Standard frameworks targeting circRNA-specific quality control and manufacturing are imminently needed.
Closing Remarks
CircRNAs represent a paradigm-shifting key to simplifying some of the most critical diseases threatening human life. Although challenges persist concerning their synthesis, quality control, delivery, and regulation, the ongoing innovations and research indicate that most of these problems will be solved by the end of this decade. Industry leaders like Merck and Orna Therapeutics are already working at the forefront of this modality. They will position it as the face of transformative healthcare in the coming years.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.