Circular RNA – Exploring New Potentials Beyond the Loop
Circular RNAs (circRNAs) are a specific group of non-coding RNAs that are present in a structural covalently closed loop and do not exhibit distinct five caps and 3poly (A) tails like linear RNAs. It was first identified as a structural element in viroids in 1976, where circRNAs lay unnoticed for nearly two decades until they were declared mere splicing byproducts. Yet, with recent advancements in sequencing technology, their prevalence and functional role in various biological processes have come to light and are being elucidated at a faster pace. CircRNAs are now recognized as key modulators of gene expression, with immense potential for diagnostic, therapeutic, and biotechnological applications.
Importance of CircRNA in Molecular Biology
Over the last couple of years, the understanding and interest in the role of circular RNAs (circRNAs) have increased significantly due to their intricate functions in gene regulation. The role of the biological activities of circRNAs in vaccine development makes them an attractive alternative to traditional linear mRNAs. The existence of a closed-loop structure is one of the main advantages of circRNA, making them more stable and less susceptible to degradation by exonucleases, unlike traditional mRNA. Furthermore, circRNAs are capable of enhancing gene expression control by functioning as efficient scaffolds for recruiting RNA-binding proteins (RBPs) and acting as micro ribonucleic acid (miRNA) sponges.
Traditional mRNA vaccines are effective, but their complex development and storage procedures make their application difficult. With circRNAs, several advantages over conventional mRNA vaccines can be easily achieved, including a longer shelf life, reduced need for cold storage, and an enhanced immune response to the vaccine. Furthermore, circRNAs can be used to more effectively carry vaccine antigens, which would elicit a strong immune response while eliminating the degradation and immune evasion risks that some mRNA constructs encounter.
The advancement in understanding circRNAs is changing the scope of gene regulation, and this could significantly transform current methods of vaccine development by making them easier, more robust, and flexible in designing for antigen delivery and immune activation.
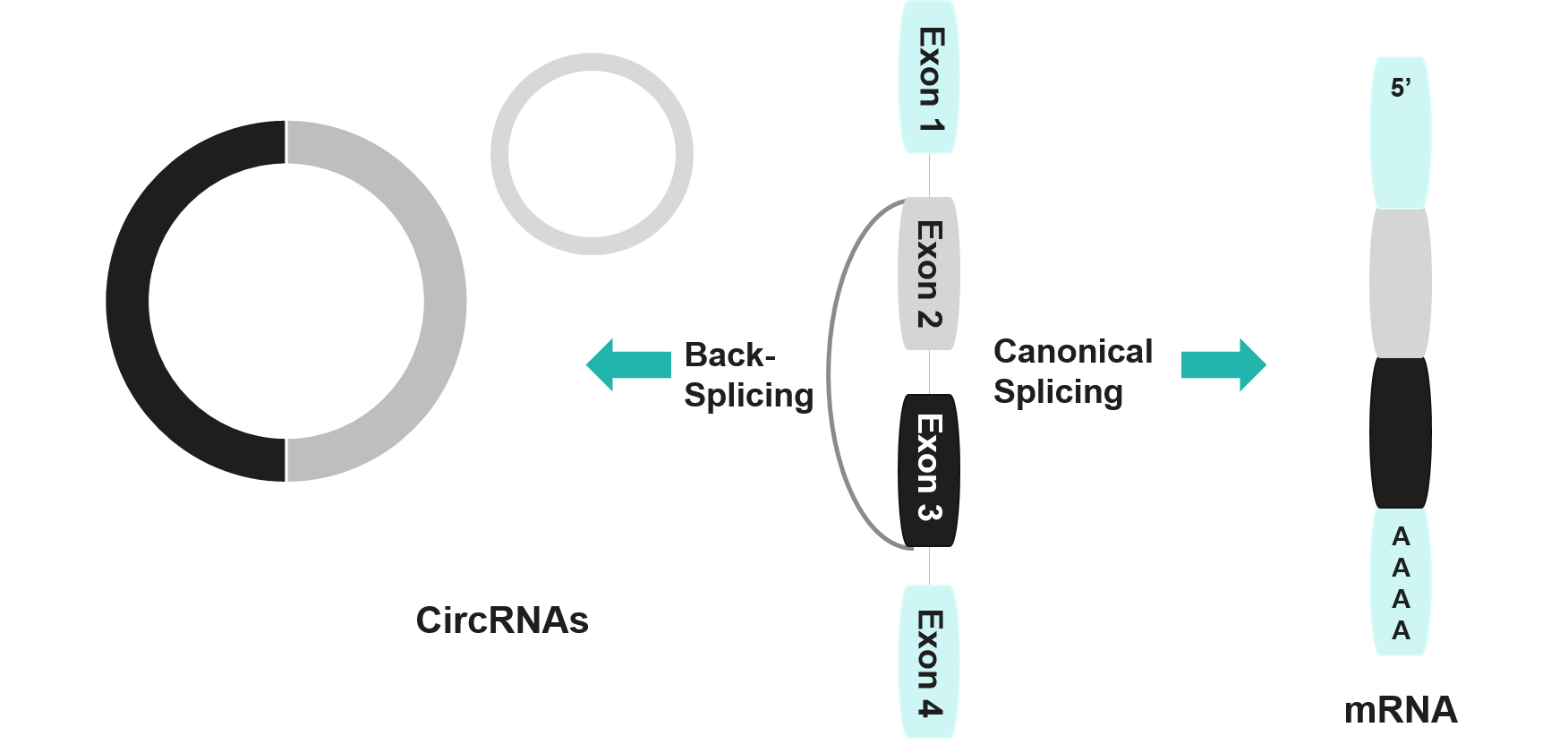
Fig 1: Structure of circRNA and mRNA
Understanding Circular RNA Biology in Vaccines
The Biological Mechanisms Involved in the synthesis of circRNAs work out when a spliceosome catalyzes a back-splice joining of a downstream 5’ splice site to an upstream 3’ splice site. This process results in the formation of a loop structure known as a closed-loop structure. This closed-loop structure is highly stable, making it favorable for use in vaccine development. The various types of CircRNA offering a more improved vaccine development include –
- Exonic circRNAs (ecRNAs) – The exonic segments of genes are the primary constituents of ecRNAs in the cytoplasm. These components are crucial for optimizing immune responses by modulating the actions of microRNA (miRNA). ecRNA functions, as miRNA sponges, could also facilitate the improvement of mRNA vaccines or the translation of protein antigens, thereby enhancing the efficacy of vaccines. The upregulated expression of these RNAs in tissues makes them particularly suitable for targeted vaccine approaches that require stringent control of immune responses.
- Intronic circRNAs (ciRNAs) – The introns of circRNAs are typically located in the nucleus. They are involved in some level of transcriptional regulation through their interaction with RNA polymerase II and the rest of the transcriptional machinery. This feature of circRNAs can be applied to regulate the expression of a defined set of antigens in the context of vaccine development. Due to their ability to silence transcriptional activity, fibrotic circRNAs may lead to tighter and more sustained expression of the antigen, thereby eliciting a robust and persistent immune response in mRNA vaccines.
- Intergenic circRNAs – The intergenic regions that do not code for specific proteins give rise to intergenic circRNAs. The roles of these circRNAs are yet to be defined. These circRNAs may offer new approaches to regulate immune responses through interactions with key elements that control immune cell activation. As non-coding circRNAs, they do not give rise to any proteins; however, they may be designed to modify cellular context or immune circuitry to enhance the efficacy of the vaccine.
Gauging the Potential Features of CircRNAs
Initially, immense potential was identified when mRNA vaccines were introduced. With the evolving field of vaccines, assessing the utility of circRNA for vaccine development opens up new horizons.
- The circular form of circRNAs provides better protection from degradation compared to linear mRNAs, making RNA transcripts less susceptible to destruction. This advantage is helpful in vaccine formulations as it may permit longer-lasting expression of the antigens, thereby mitigating the need for booster shots and enhancing immunity for a longer duration.
- CircRNAs have been proposed to be expressed in some tissues, suggesting that they may have specialized functions in multicellular activities. Unlike other types, these may be targeted for particular tissues, which in turn would induce more potent immune responses. For instance, circRNAs may be aimed at enhancing antigen presentation in specific immune cells to ensure an effective vaccine response.
- Conserved circRNAs have been identified in several other species, indicating that they play a significant role in evolution. This suggests that cross-platform dicRNAs may be developed as a vaccine for use in both anthropogenic and veterinary medicine, thereby boosting the possibility of widespread use in vaccine creation.
Functions of Circular RNA
The circular RNA in focus has been assessed for its ability to perform various functions, making it a favorable vaccine candidate.
miRNA Sponges
- CircRNAs are designed to bind subsequent miRNAs. Meanwhile, they are specifically engineered to block the effect of miRNAs by binding to the seed regions and reducing their interaction with mRNAs.
- This “sponge” effect leads to enhancing or suppressing the expression of genes that are regulated by the miRNA.
- For example, CDR1 and circSRY, a prominent circRNA identified as miRNA sponges, interfere with the pathway that accelerates cancer and neurodegenerative diseases.
Regulation of Transcription and Splicing
- CircRNA interacts with RNA Pol II and other transcription machinery to regulate gene expression.
- It influences splicing by competing with pre-mRNA splicing, thereby impacting the final mRNA product.
- Some circRNAs form R-loops with their DNA templates or RNA, forming a DNA-RNA hybrid, which results in alterations to the splicing of pre-mRNA and produces different protein isoforms.
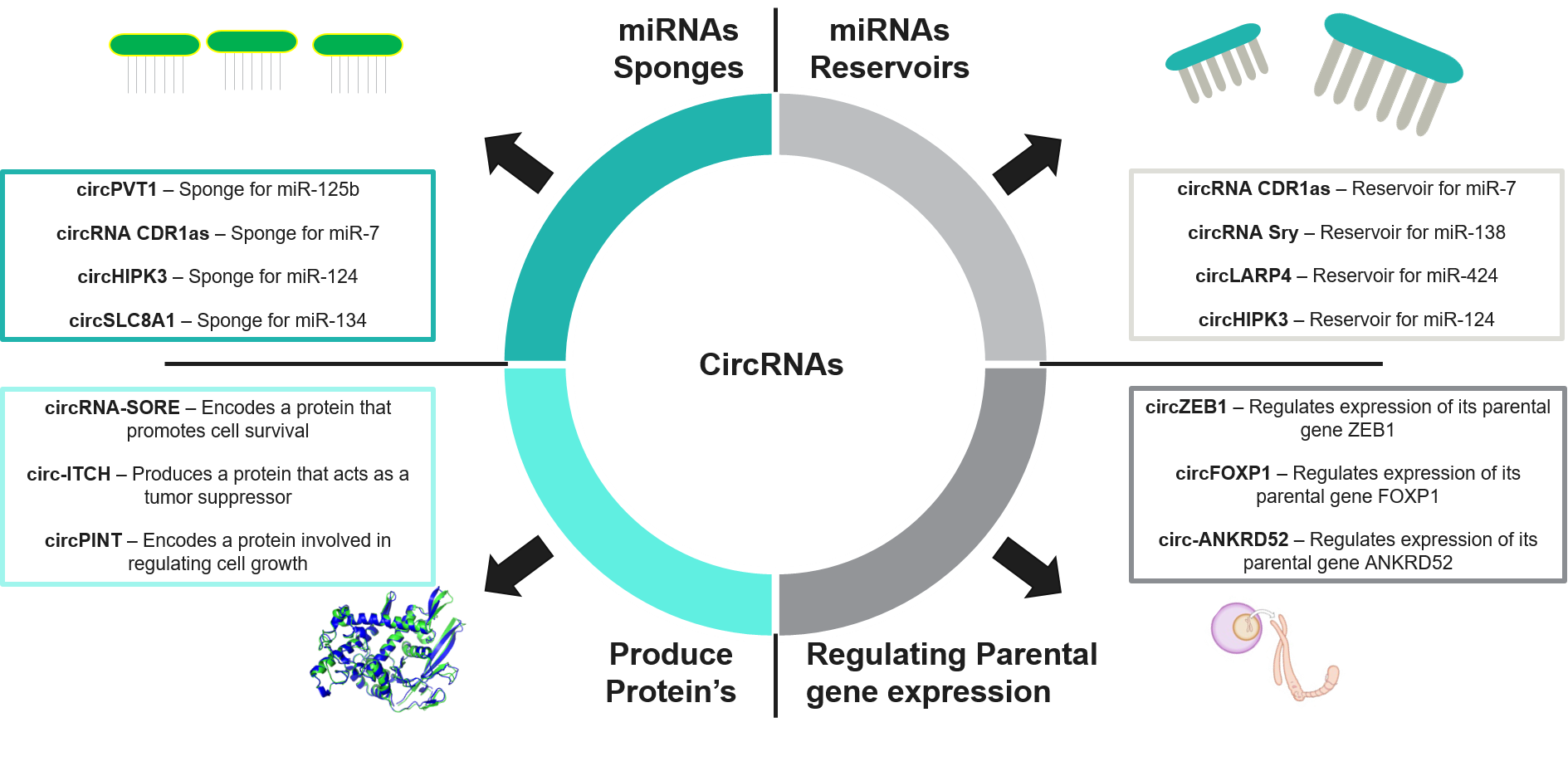
Fig 2: Functions of circRNAs
CircRNA in Disease Mechanism
These non-coding RNAs with a covalently closed-loop structure are blooming in their application with high stability and resistance to degradation. They have been the subject of intense interest because they are implicated in numerous diseases, including cancer, neurological diseases, and cardiovascular diseases. The following discussion outlines their roles in these diseases.
CircRNAs in Cancer
- Oncogenic Activities: CircRNAs function as ceRNAs or miRNA sponges in cancer suppression, as CircPVT1 promotes gastric cancer cell proliferation by sequestering miR-125, thereby enhancing its oncogenic activity. Moreover, in renal cell carcinoma, CircSDHC acts as a sequestrant for miR-127-3p, which suppresses tumour activity and enhances malignant growth.
- Diagnostic and Prognostic Value: Circular RNAs are emerging as promising biomarkers due to their stability and tissue-specific expression patterns. For example, CircHIPK3 has been highlighted with its function as a miRNA sponge, with its expression involved in the development of liver cancer as well as therapeutic drug response. Furthermore, it has recently been found that CircMRPS35 and CircLRIG3 are actively involved in the growth progression of liver cancer cells and drug resistance.
CircRNAs in Neurological Disease
- Alzheimer’s Disease: CircHOMER1 is widely enriched in the orbitofrontal cortex of the brain and regulates synaptic proteins involved in neuronal communication. Reducing the level of circHOMER1 disrupts cognitive flexibility and alters synaptic gene expression in Alzheimer’s patients, which suggests its role in synaptic malfunction and disease progression.
- Parkinson’s Disease: CircSLC8A1 interacts with the α-synuclein protein, influencing oxidative stress, aberrant transcription regulation, cell apoptosis, and autophagy in the pathology of Parkinson’s disease. This interaction aggravates neuronal toxicity and makes circSLC8A1 a potential therapeutic target for breakthroughs against Parkinson’s disease.
CircRNAs in Cardiovascular Disease
- Atherosclerosis: CircANRIL modulates the maturation of ribosomal RNA, which in turn influences the growth of vascular smooth muscle cells and the stability of atherosclerotic plaques. This indicates its participation in the progression of atherosclerosis.
- Heart Failure: CircNFIX acts as a miR-145 sponge to facilitate cardiac hypertrophy and aggravate heart failure. Consequently, it presents itself as a candidate for therapeutic intervention to prevent cardiac remodelling.
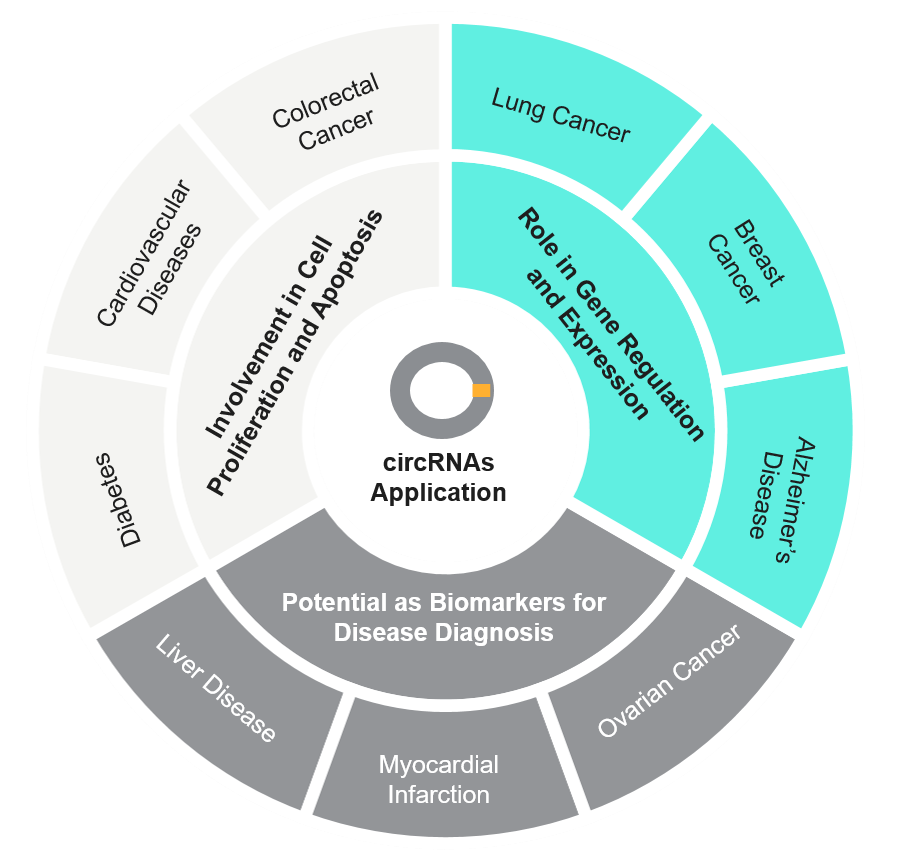
Fig 3: Potential Areas of Application of circRNAs
Ecosystem
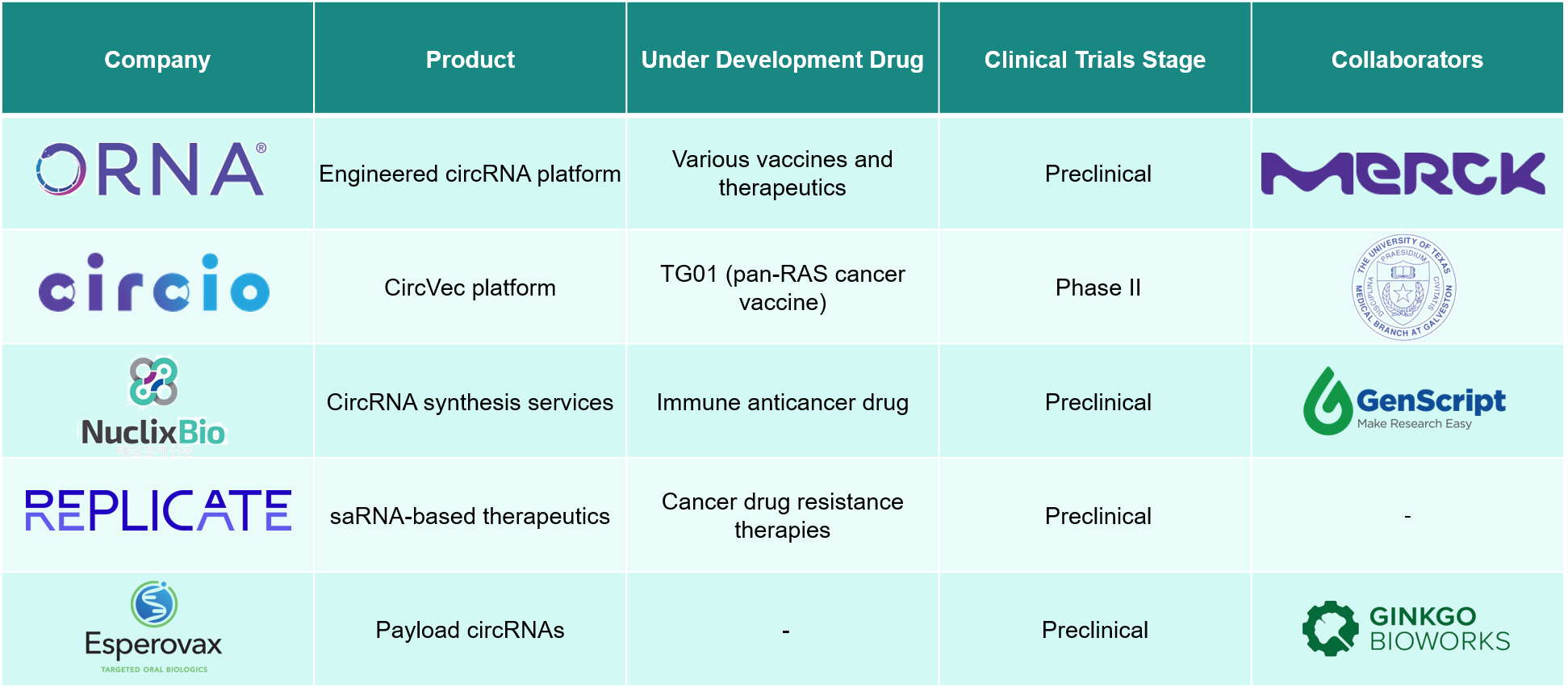
The rapidly evolving ecosystem in the arena of circRNA as a potential vaccine candidate has observed Orna Therapeutics as one of the most promising players, wherein it specializes in engineered circular RNA (oRNA) therapeutics. Circio AB is a biotechnology firm creating circular RNA medicines for cancer and other diseases, utilizing its CircVec platform. The following are other prominent players.
Today’s Challenge and Tomorrow’s Direction
Despite the promise of circRNA research in maintaining effective therapeutics, there are several challenges remaining as a hurdle.
- Methodological and Technical Barriers – The identification, detection, and quantification of circRNAs still remain challenging due to their circular nature, and the differentiation between active and non-active circRNAs requires more advanced technologies.
- Understanding CircRNA Functionality in Different Contexts – Various strategic studies are required to elucidate and establish the roles of circRNAs across different tissues and cellular developmental stages, where it has become challenging to deplete or generate the circular form of a gene without affecting its linear counterpart.
Conclusion
Circular RNAs possess an intriguing advantage in molecular biology, with profound implications for gene regulation and disease processes. Their novel characteristics make them promising candidates for diagnosis and therapy. With increasing understanding of the biology of circRNA, the medical potential is vast. Future studies will undoubtedly provide new insights into the function of circRNA in disease and health, as well as establish novel clinical applications.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.