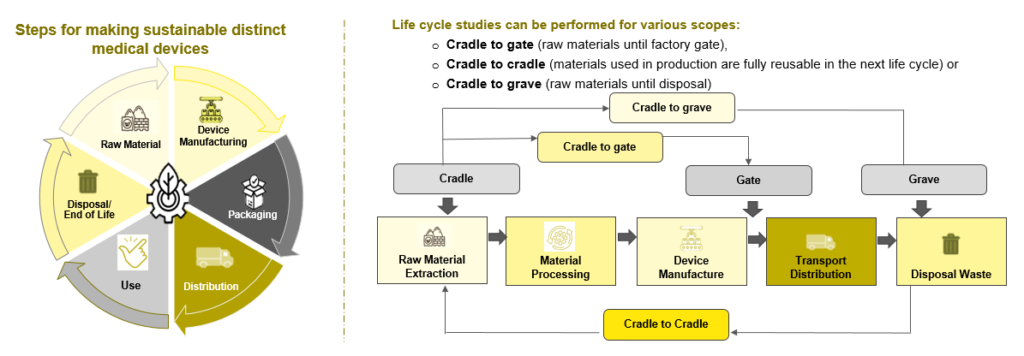
Sustainability evaluates and applies best practices to increase efficiency, reduce waste, reduce energy and water use, and create better products. Moreover, the environmental impact of a product is assessed at all stages of its life cycle. It includes the extraction and processing of raw materials, production, distribution, use, recycling and final waste. LCA (Life Cycle Analysis/Assessment) is the term for this process. Also, LCA is the best tool for calculating the environmental impact of a system. Without this, the true environmental impact is unknown.
The safety of medical devices can be improved through a number of strategies, including material selection, packaging, manufacturing processes, end-of-life treatments and packaging. Every modification made to the equipment could have unanticipated consequences for other components. Before making any changes, product engineers must consider how such changes can impact the performance, usability, safety, and other requirements of a medical device.
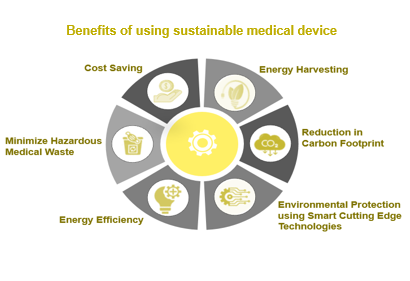
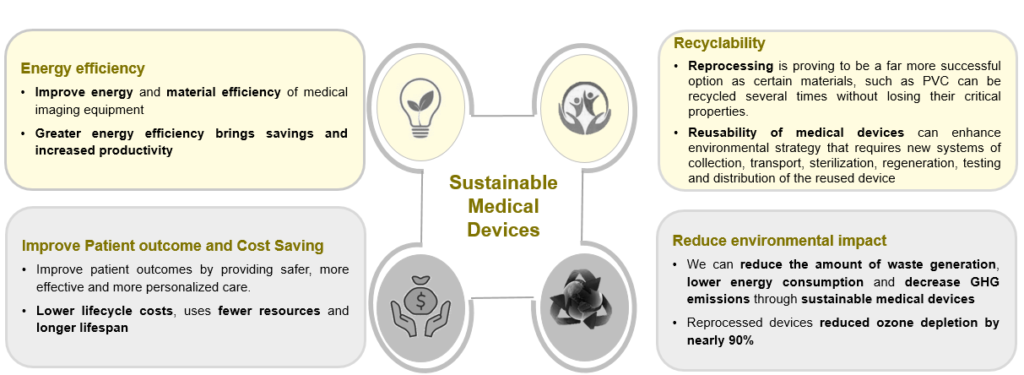
Sustainable medical devices not only benefit the environment and consumers, but they may also reduce expenses, draw in investors, improve a company’s brand, and provide it a competitive edge.
Sustainable Design for Analysis in Medical Devices
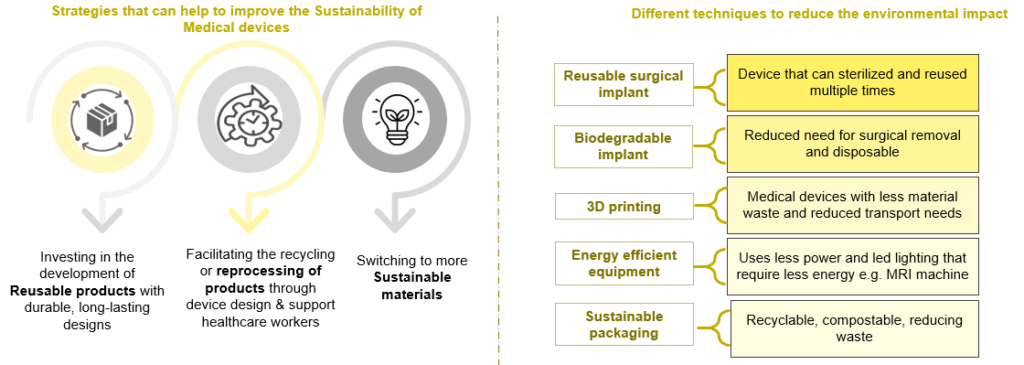
A sustainable new product design requires less raw material extraction, produces less waste and focuses on the 4R loops: reuse, repair, remanufacture and recycle, all of which extend the lifespan of products, offering environmental and economic upsides. Three activities comprise the environment framework method
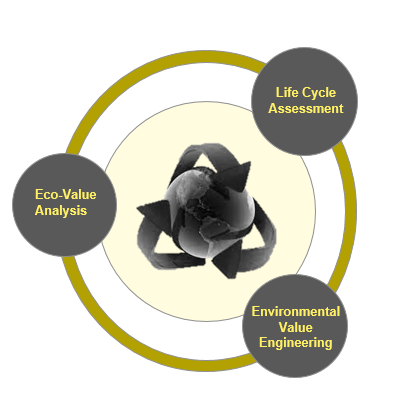
Lifecycle Assessment: Determine a medical device’s environmental impact by analyzing multiple parameters, including CO2 emissions, energy and water consumption, recycling rate (percentage of material recycled) and recovery rate (percentage of material used to generate energy when the product reaches the end of life).
Eco-value Analysis (on existing products): It is possible to envision how a medical product could be better designed to improve the environmental impact and economic value of the materials, parts, and product. Existing medical devices, already in-market, can be analyzed against the following criteria such as materials used, product durability, energy efficiency, ease of disassembly & reassembly and ease of maintenance & repair.
Environmental Value Engineering (on new products): The product development process for a new medical design guarantee compliance with specific environmental regulations, minimize carbon footprint (i.e. carbon footprint lower than a predefined target), enable circular economy (design for reuse, repair, recycling) and sustainable packaging.
Sustainability Trends in Medical Devices Industry
Manufacturing
Material Selection: Replacing existing biomaterials with renewable resources such as cellulose based or plant based, renewable polyethylene (PR) & polyethylene terephthalate (PET), etc. E.g. American start-up Lia launched FDA approved biodegradable pregnancy test, made up of natural plant fibers that breaks down when flushed.
Device Design: Achieve “zero defect”, waste-free manufacturing. Reduce the size or weight of products to reduce material consumption. Integrate wireless technology to make the device portable.
Manufacturing Process: Use 3D printing/additive manufacturing to reduce waste. Reduce drinking water, increase energy efficiency, reuse, increase efficiency, reduce chemical use. For e.g. Elos Medtech invested in a machine that recycles cutting oil.
Advanced Technologies: Battery-free medical devices that use electromagnetic radiation (Rfeh) or body heat and light to power the device. For e.g. Battery-free, wireless, dual-channel EEG system developed by the Nanoelectronics Research Center IMEC in collaboration with the Holst Center.
Packaging
Alternatives to Packaging Elements: Use reusable packaging or convenience packaging. Eliminate the use of plastic containers or gasoline foam. For e.g. DuPont’s Tyvek features a lightweight, single material made from certified high-density polyethylene.
Distribution:
Medical Supply Chains: Digitizing inventory management will make it possible to recycle the most important items.
Disposal and Recycling:
Medical Waste Management: Use of practices for carbon capture and disposal of waste streams. E.g. US-based startup Trilogy MedWaste provides services for the management of healthcare waste and its compliance.
Reusability and Recycling: Pulp bedpans and urinals made from 100% recycled over-issued newspaper.
Future Impact of Sustainable Medical Devices
Climate change is being discussed worldwide, with a focus on reducing its impacts and improving waste management. The healthcare sector is detrimental to the environment and is mostly to blame for the majority of environmentally hazardous practices. Moreover, medical devices manufacturers can become more sustainable by putting strategies into place. It includes establishing sustainable manufacturing processes, collaborating with healthcare institutions and other welfare systems to facilitate the adoption of sustainable workflows and technologies, and investing in service models.
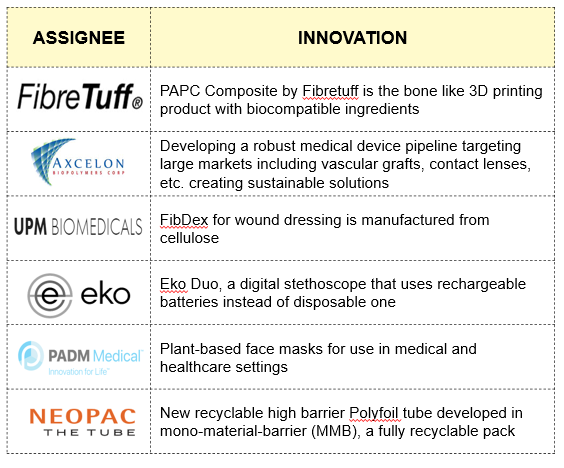
Recent Innovations
Key Market Happenings
- Amcor has made significant progress toward sustainability with the launch of new environmentally friendly medical laminates.
- To produce a sustainable silk protein that may be used in manufacturing medical devices, Evonik partnered with the German biotech startup AMSilk.
- Johnson and Johnson, Ethicon’s parent company, worked together with Eastman. This partnership made it possible to package medical devices with co-polyester made from recycled materials.
- AMNOVIS and BAAT Medical collaborated to create an innovative workflow for 3D-printed medical devices.
- Technology Scotland and Heriot-Watt University announced a partnership to help Scotland’s SMEs have faster access to technology design capabilities in order to improve design requirements, with a special emphasis on the sustainability of medical devices.
- Endoscopy Products LLC and AA Medical, a developer of sustainable solutions, have partnered to establish a leading medical device reprocessing business that caters to the endoscopy, orthopedic, and other surgical device sectors.
- A collaboration was formed between Solvay and Ostium to recycle single-use surgical instruments.