Opportunity Mapping – At-home Diagnostics
A leading diagnostics company sought to expand into the at-home testing segment, targeting aging populations (55+ years) in the US, EU, and Japan, focusing on cardiovascular, kidney, and metabolic health.
While the company already had point-of-care and lab-based biomarker solutions, leadership sought to evaluate whether a combined at-home panel could be a commercially viable and clinically relevant addition to their portfolio.
The client’s Strategy and Innovation teams wanted to:
- Validate commercial attractiveness and adoption readiness of such multiplex panels across the US, EU, and Japan
- Identify specific user segments (aging at-risk, chronic disease management, preventive wellness) with the highest willingness to adopt
- Map regulatory, technological, and partnership gaps that could impact go-to-market feasibility
Engagement Objective:
The client engaged the consulting team to deliver a data-driven opportunity validation, aiming to:
- Quantify the total addressable market (TAM), serviceable available market (SAM), and growth trajectory of at-home diagnostics in cardiovascular, kidney, and metabolic testing
- Map consumer and physician adoption drivers and barriers
- Benchmark existing offerings and emerging innovations in biomarker detection technologies
- Develop go/ no-go commercial recommendations, including target segments, partnership models, and investment priorities
Methodology:
A three-phase research and validation process was deployed, combining secondary intelligence, stakeholder interviews, and market modeling:
| Phase | Description | Deliverables |
| 1 | Market Landscape Definition & Segmentation | Mapped the current at-home diagnostics ecosystem, segmented aging populations by disease burden and adoption readiness, and identified key clinical and payer trends shaping cardiovascular, renal, and metabolic testing demand. |
| 2 | Opportunity Validation & Stakeholder Voice | Validated unmet needs and adoption barriers through expert interviews, consumer sentiment analysis, and regulatory benchmarking across the US, EU, and Japan markets. |
| 3 | Commercial Feasibility & Opportunity Pathways | Built market adoption scenarios, benchmarked solution archetypes, and prioritized market entry models with quantified go/ no-go recommendations. |
Client Impact:
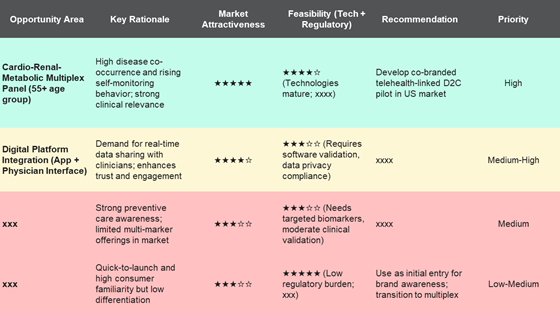
- Decision Clarity: Our models estimated a $1.2 billion USD addressable market opportunity by 2030, segmented by adoption scenario (wellness, telehealth-linked, clinical).
- Portfolio Focus: Enabled Client to prioritize Cardio-Renal-Metabolic multiplex panel as the lead concept among 5 initially considered at-home test ideas.
- Investment Confidence: Helped secure internal go-ahead for pilot development and partnership discussions with two telehealth providers and one microfluidic technology vendor.
- Regulatory Roadmap Alignment: Outlined CLIA-waived (US), IVDR compliance (EU), and PMDA compliance (Japan) pathways, de-risking early feasibility planning.
- Commercial Readiness Blueprint: Delivered actionable GTM sequencing – D2C pilot in the US → Hybrid telehealth model → EU regulatory expansion → Japan entry through local diagnostic partnerships and regulatory harmonization.
Outcome Snapshot:
Every at-home test looks promising on paper until users, payers, and regulators weigh in. Partner with us to stress-test your opportunity with real-world data and adoption signals.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.