Seawater Electrolysis: Sustainable Hydrogen Production
Seawater electrolysis presents an innovative approach to harnessing hydrogen energy using seawater as an electrolyte. In this process, an electrolyzer is deployed to split water molecules into oxygen and hydrogen gases by applying an electric current. One of the vital benefits of seawater electrolysis is its independence from freshwater resources, which are becoming scarce. Hybrid seawater splitting, direct seawater electrolysis, and impurity removal techniques have shown promise in enhancing the efficiency and viability of this technology.
Moreover, remarkable progress has been made in developing high-efficiency electrocatalysts for seawater splitting, opening new possibilities for more efficient hydrogen production. Additionally, researchers have explored performance modeling to understand the impact of current density and seawater salinity on the electrolysis process.
Advantages of Seawater Electrolysis:
1. Abundance and Availability
Seawater is abundant and widely available, making it a cost-effective and easily accessible resource for electrolysis. This eliminates the need for freshwater sources, which are becoming increasingly scarce.
2. Integration with Renewable Energy:
Seawater electrolysis can be carried out with renewable energy sources, including offshore wind and solar power. This integration reduces transportation and distribution costs, making green hydrogen more affordable and environmentally friendly.
3. Scalability:
The vast amounts of seawater available allow for the scalability of seawater electrolysis to meet the increasing demand for hydrogen. Also, this can potentially reduce reliance on fossil fuels and mitigate the effects of climate change.
4. Lower Capital Costs:
Seawater electrolysis offers the potential for lower capital costs compared to desalinated water electrolysis. This is due to the natural elimination of waste brine, which is only slightly enriched with salts, reducing the need for additional treatment processes.
5. Reduction Of Waste:
Seawater electrolysis eliminates the need for desalination, an energy-intensive process with environmental impacts. By utilizing seawater directly, the process reduces waste and minimizes the overall ecological footprint.
6. High Reserves:
Seawater has abundant resources, making it a favorable choice for large-scale hydrogen production. This inherent advantage of seawater electrolysis contributes to its potential as a sustainable and long-term solution.
Seawater Electrolysis Cost Compared To Freshwater Electrolysis Cost
In the realm of research and literature, the cost comparison between seawater electrolysis and freshwater electrolysis has garnered significant attention. While some variations may exist depending on specific factors and technologies, a generous exploration reveals intriguing insights:
1. Potential For Lower Capital Costs:
Seawater electrolysis promises lower capital costs than freshwater electrolysis. The natural elimination of waste brine, only slightly enriched with salts, alleviates the need for extensive additional treatment processes. Also, this inherent advantage could pave the way for more cost-effective implementation of seawater electrolysis systems.
2. Reduced Cost of Water Production:
In the grand scheme of electrolysis, the cost of producing water with the requisite quality stands lower than the cost of electricity to operate the electrolyzer. Seawater’s bountiful and widely available nature allows for its direct utilization as an electrolyte, bypassing the necessity for elaborate water treatment processes. This streamlined approach contributes to cost reduction and overall efficiency.
3. Abundance and Wide Availability:
One of the most compelling advantages of seawater electrolysis lies in seawater’s abundance and wide availability. This cost-effective resource renders the reliance on freshwater sources unnecessary, thereby mitigating potential costs related to extraction, treatment, and transportation. By harnessing the readily available seawater, electrolysis becomes more economically feasible and environmentally friendly.
Challenges of Seawater Electrolysis:
Here are a few notable challenges discovered in seawater electrolysis:
1. Chlorine Crossover:
A notable challenge in seawater electrolysis arises from salt and impurities, which can lead to undesirable side reactions and corrosion. Traditional electrolysis may produce toxic and corrosive chlorine ions, threatening catalysts and electrodes. To mitigate this, ongoing efforts focus on enhancing catalyst durability and extending the electrolyzer’s lifetime.
2. Corrosion Concerns:
The diverse array of salts and impurities in seawater poses a risk of corrosion within the electrolyzer system. Chloride ions and other corrosive substances can erode electrodes and system components, potentially impacting the efficiency and longevity of the electrolysis process. Rigorous research endeavors strive to develop corrosion-resistant materials and innovative protective measures.
3. High Cell Voltages:
Seawater electrolysis typically demands higher cell voltages than freshwater electrolysis due to the elevated conductivity of seawater. This disparity translates to increased energy consumption and associated costs. Innovations in cell design and enhanced power management techniques are in progress to address this challenge and optimize energy utilization.
4. Electricity Consumption:
Due to its heightened conductivity and impurity content, seawater electrolysis can be more energy-intensive than freshwater electrolysis. This discrepancy results in elevated electricity consumption and financial implications. Pioneering advancements delve into energy-efficient strategies and resourceful filtration technologies to alleviate this concern.
5. Impurity Management:
Seawater harbors impurities such as suspended solids and organic matter that can impede the electrolyzer’s performance and efficacy. To ensure optimal operation and prevent fouling or clogging, meticulous impurity management and advanced filtration systems must be implemented.
6. Catalyst Development:
The quest for efficient, stable, and selective catalysts for seawater electrolysis poses a considerable challenge. Seawater’s unique composition, coupled with the presence of impurities, can influence catalyst performance and longevity. Tirelessly, researchers embark on ongoing endeavors to discover catalyst formulations that can unlock the true potential of seawater electrolysis.
Recent Advances In Seawater Electrolysis
Seawater electrolysis has made significant advances in recent times. Here are a few mentioned below:-
1. Electrocatalysts:
Various high-efficiency electrocatalysts have made significant progress in splitting seawater and shown potential. These include metal oxides and hydroxides, metal phosphides, metal nitrides, and metal borides.
2. Anode Design Strategies:
A review published in Wiley Online Library highlights recent developments and future perspectives in anode design strategies for seawater electrolysis. Strategies such as electronic modulation, oxygen vacancies creation, and amorphous materials are discussed.
3. Direct Seawater Electrolysis:
A study was published on the direct electrolysis of real seawater without alkalization or acidification. The study achieved long-term stability exceeding 100 hours at 500 mA cm–2 and similar performance to a typical proton exchange membrane (PEM) electrolyzer operating in high-purity water. The study utilized Lewis acid-modified electrodes (Cr2O3–CoOx) in a flow-type natural seawater electrolyzer.
4. Discarding Impurities:
Researchers are working to enhance electrolyzer efficiency and longevity in seawater electrolysis. Contaminants in seawater can cause side reactions and corrosion, shortening the device’s lifespan. Moreover, innovative solutions, like removing salts and impurities, are being explored to ensure sustainable hydrogen production. Furthermore, advancements in electrolysis promise a cleaner and more renewable energy future.
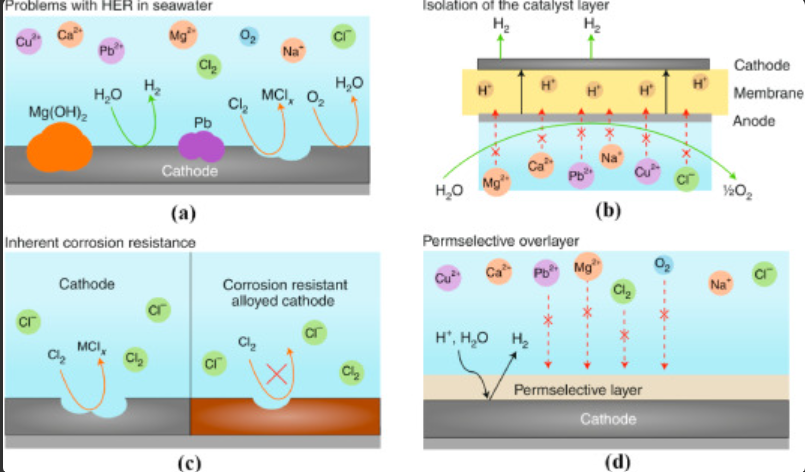
Image: Explaining recent advances in electrolysis
Electrocatalysts For Seawater Electrolysis
Electrocatalysts, as key players in seawater electrolysis, assume a pivotal role by facilitating the vital splitting of water molecules into two gases, viz hydrogen and oxygen. Remarkable advancements in seawater electrolysis have been observed, with the spotlight on the following electrocatalysts:
1. Metal Oxides and Hydroxides:
Extensively studied for the oxygen evolution reaction (OER) in seawater electrolysis, metal oxides, and hydroxides have showcased tremendous potential. Moreover, among these are nickel oxide (NiO), cobalt oxide (CoOx), and iron oxide (Fe2O3), demonstrating remarkable catalytic properties.
2. Metal Phosphides:
Intriguingly, metal phosphides like nickel phosphide (Ni2P) and cobalt phosphide (Co2P) have emerged as promising electrocatalysts for the OER. Their outstanding catalytic activity and steadfast stability under harsh seawater conditions have piqued researchers’ curiosity.
3. Metal Nitrides:
The investigation into metal nitrides as electrocatalysts for the hydrogen evolution reaction (HER) and OER in seawater electrolysis has been fruitful. Molybdenum nitride (MoN) and tungsten nitride (WN) have emerged as standouts, boasting excellent catalytic activity and unwavering stability.
4. Metal Borides:
Metal borides such as nickel boride (Ni2B) and cobalt boride (Co2B) have captured attention by exhibiting exceptional performance in the HER for seawater electrolysis. Their high catalytic activity and resilience contribute to their promising role in the electrolysis process.
Performance Modeling Of Seawater Electrolysis
Generously exploring the realm of seawater electrolysis reveals captivating findings from recent research endeavors, shedding light on key aspects that enhance its efficiency and performance:
1. Performance Modeling:
Dedicated researchers have meticulously studied performance modeling in seawater electrolysis, delving into the effects of current density and seawater salinity. Four theoretical models were developed through a publication, intricately grounded in physical and chemical principles. These models are indispensable tools for investigating the process’s efficiency and performance.
2. Anode Design Strategies:
Innovative anode design strategies have been the subject of focused study in elevating seawater electrolysis performance and efficiency. A comprehensive review, documented in Wiley Online Library, embraces recent developments and exciting future perspectives in this domain. Strategies like electronic modulation, oxygen vacancy creation, and amorphous materials emerge as promising pathways for optimizing the anode design.
Promising Results For Cost-Effective And Sustainable Hydrogen Production
The latest discoveries paint a hopeful picture for seawater electrolysis as a viable, cost-effective, and sustainable method for hydrogen production. Let’s take a glimpse at the promising results that illuminate our journey toward a greener and more harmonious energy landscape:
1. Scaling Up For Cost Reductions:
As we venture into scaling up green hydrogen plants to the impressive capacity of 20MW and beyond, a world of possibilities unfolds. Recent analyses reveal that such scaling efforts could lead to a remarkable reduction of approximately 30% in operation and maintenance costs. The threshold of three-to-four-megawatt size projects is projected to be the tipping point, rendering hydrogen plants significantly cheaper to install. This advancement paves the way for enhanced cost-effectiveness and accessibility of green hydrogen technologies.
2. Metal-Free Catalysts For Sustainability:
Researchers at the esteemed University of Surrey have revealed the potential of metal-free catalysts. These catalysts hold the key to developing cost-effective and sustainable hydrogen production technologies. With this innovative approach, we could potentially reduce the reliance on metal catalysts, which are energy-intensive to mine and manufacture. Such a shift also aligns beautifully with our commitment to creating a more sustainable and eco-friendly future.
3. Lowering Electrolyzer Costs Through Innovation:
The International Renewable Energy Agency (IRENA) presents a visionary report that outlines strategies to reduce electrolyzer costs through continuous innovation, performance improvements, and strategic upscaling. Additionally, with renewable power costs steadily declining and progressive advancements in electrolyzer technologies, the trajectory is set for “green” hydrogen to emerge as a cost-competitive solution by 2030. This exciting development holds promise for a future where clean hydrogen is pivotal in our global energy landscape.
4. Abundant Renewable Resources:
The allure of green hydrogen production lies in the markets graced with abundant and low-cost renewable resources. Notably, regions like the Middle East, Africa, Russia, the US, and Australia stand poised to produce green hydrogen at the remarkable price range of €3 to €5 per kilogram today. This abundance of renewable resources ignites a beacon of hope for the widespread adoption of sustainable and accessible green hydrogen solutions.
Conclusion
In the realm of sustainable hydrogen production, seawater electrolysis emerges as a beacon of promise. Recent advancements in this field have showcased remarkable progress and boundless potential. The development of theoretical models has provided invaluable insights into the impact of current density and seawater salinity on the process’s performance. Moreover, with each stride forward, the vision of cost-effective and sustainable hydrogen production using seawater as an electrolyte shines brighter. These collective advancements affirm the untapped potential of seawater electrolysis, fueling our journey toward a greener and more sustainable energy landscape. Also, as we chart a course toward a future empowered by clean and renewable hydrogen, the allure of seawater electrolysis beckons us toward a world where sustainable energy solutions become a tangible reality.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.



