TROP-2 Directed Antibody-Drug Conjugates: Clinical Pipeline
In the last decade, ADCs (Antibody-Drug Conjugates) have emerged as a class of drug therapies to treat cancer by combining potent anti-cancer drugs (i.e., payloads) with specific monoclonal antibodies that deliver anti-cancer drugs to the tumor site. ADCs include three important portions comprising a monoclonal antibody, a linker (organic spacer), and an anti-cancer drug. ADCs can deliver anti-cancer drugs directly to the inside of tumor cells so they do not damage the nearby healthy cells.
Current Scenario for ADCs
The therapeutic efficacy of the ADC molecule is slightly based on the successful cleavage of selective linkers and stability to deliver the payload. Most approved ADC products are in lyophilized powder formulations due to concerns about product stability in the liquid dosage form. Biopharmaceutical Company’s research has recently focused on developing emerging ADCs, including bispecific ADCs, immune-stimulating ADCs, and dual-drug ADCs, to enhance tumor specificity and heterogeneity with enhanced activity. Two types of linkers, such as cleavable and non-cleavable, are used in developing ADCs. These linkers have essential roles in the pharmacokinetics, therapeutic index, and favorable outcomes of the ADC. A number of well-established anticancer drugs are used as payloads in the development of ADC. DNA topoisomerase 1, microtubules, and hDNA are key therapeutic targets for the payloads present in antibody-drug conjugates.

Figure 1: Components of Antibody-Drug Conjugates
Trop-2 and Its Expressions
TROP2 (Trophoblast cell surface antigen 2) is tumor-associated calcium signal transducer 2 (TACSTD2). This cell surface glycoprotein is present as a transmembrane transducer of intracellular (IC) calcium signals. It is encoded by the TACSTD2 gene located on the 1p32 chromosome. TROP2 is overexpressed in multiple tumor cells due to its importance in cellular functions such as cell proliferation, invasion, migration, and apoptosis of cells. Multiple research institutes have validated the TROP-2 as a therapeutic target for treating different types of cancer. Research studies show that TROP2 mRNA may be detected in tissues such as the breast, lungs, ovaries, colon, kidneys, and keratinocytes. Many research papers have validated that TROP2 is overexpressed in multiple types of tumor cells associated with breast, colorectal, pancreatic, gastric, and ovarian cancers compared with the respective normal tissue. Currently, TROP2 is a promising therapeutic target for multiple types of cancer, including advanced metastatic cancers.
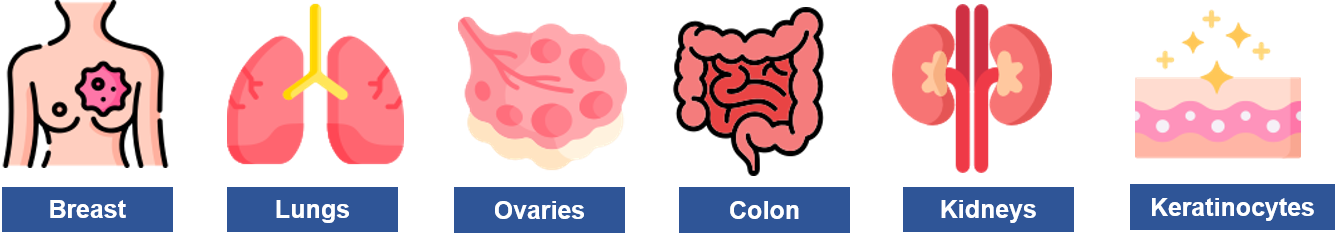
Figure 2: TROP-2 Expressions in Cancer

Figure 3: Indications in Trop-2 Targeting ADC
FDA Approved Product: Sacituzumab Govitecan (Trodelvy)
Since the first ADC (Mylotarg® – gemtuzumab ozogamicin) was approved in 2000 by the US Food and Drug Administration (FDA), 14 ADCs have received market approval worldwide. The first Trop-1 targeting ADC, i.e., Sacituzumab govitecan (Trodelvy), got FDA approval in April 2020 for the treatment of breast cancer. Since then, multiple biopharma companies are developing other novel ADCs that target Trop-1 to treat various types of cancers. Sacituzumab govitecan (IMMU-132) contains SN-38 (a metabolite of irinotecan) as a payload and anti-Trop-2 antibody. It can be used for the treatment of multiple cancers such as breast cancer (triple negative breast cancer), small cell lung cancer, etc. Trodelvy is administered to cancer patients in a continuous 21-day treatment cycle as an intravenous infusion, wherein the dosage is given once weekly on days 1 and 8 of the cycle. Trodelvy can cause adverse effects such as nausea, vomiting, diarrhea, fatigue, constipation, alopecia, anemia, and abdominal pain. Sacituzumab govitecan (Trodelvy) was developed by Immunomedics and later acquired by Gilead Sciences. Trodelvy is present in lyophilized powder in single-dose vials for intravenous infusion, and the recommended dose is 10 mg/kg. However, there are several side effects associated with Trodelvy, wherein the common ones include decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, diarrhea, nausea, vomiting, decreased lymphocyte count, fatigue, alopecia, and constipation.
Figure 4: Sacituzumab govitecan (Trodelvy)
Clinical Pipeline Targeting TROP-2
So far, around thirteen active antibody-drug conjugates targeting Trop-2 are under clinical development. Below are some highlights on Trop-2 targeting antibody-drug conjugates.
- In total, thirteen active antibody-drug conjugates targeting Trop-2 are under clinical development, out of which two ADCs (Datopotamab Deruxtecan & SKB264) are in phase III trials, two ADCs (SHR-A1921 & DB-1305) are in phase II trials and nine ADCs are under phase I trials
- Daiichi Sankyo and AstraZeneca highlighted that the biologics license application (BLA) for datopotamab deruxtecan (Dato-DXd) has been accepted in the US. Dato-DXd will be used for the treatment of adult patients with (HR)-positive, HER2-negative breast cancer. It is expected to get a regulatory decision by the first quarter of 2025.
- Merck did a licensing agreement with Sichuan Kelun Pharmaceutical for its phase III asset SKB264 for $1.4bn to compete with Daiichi Sankyo and AstraZeneca’s datopotamab deruxtecan (Dato-DXd)
- Alphamab Oncology has developed a new generation bispecific ADC that targets TROP2 and HER3 to treat advanced malignant solid tumors
- Key indications include triple-negative breast cancer, non-small cell lung cancer, urothelial cancer, and solid tumors
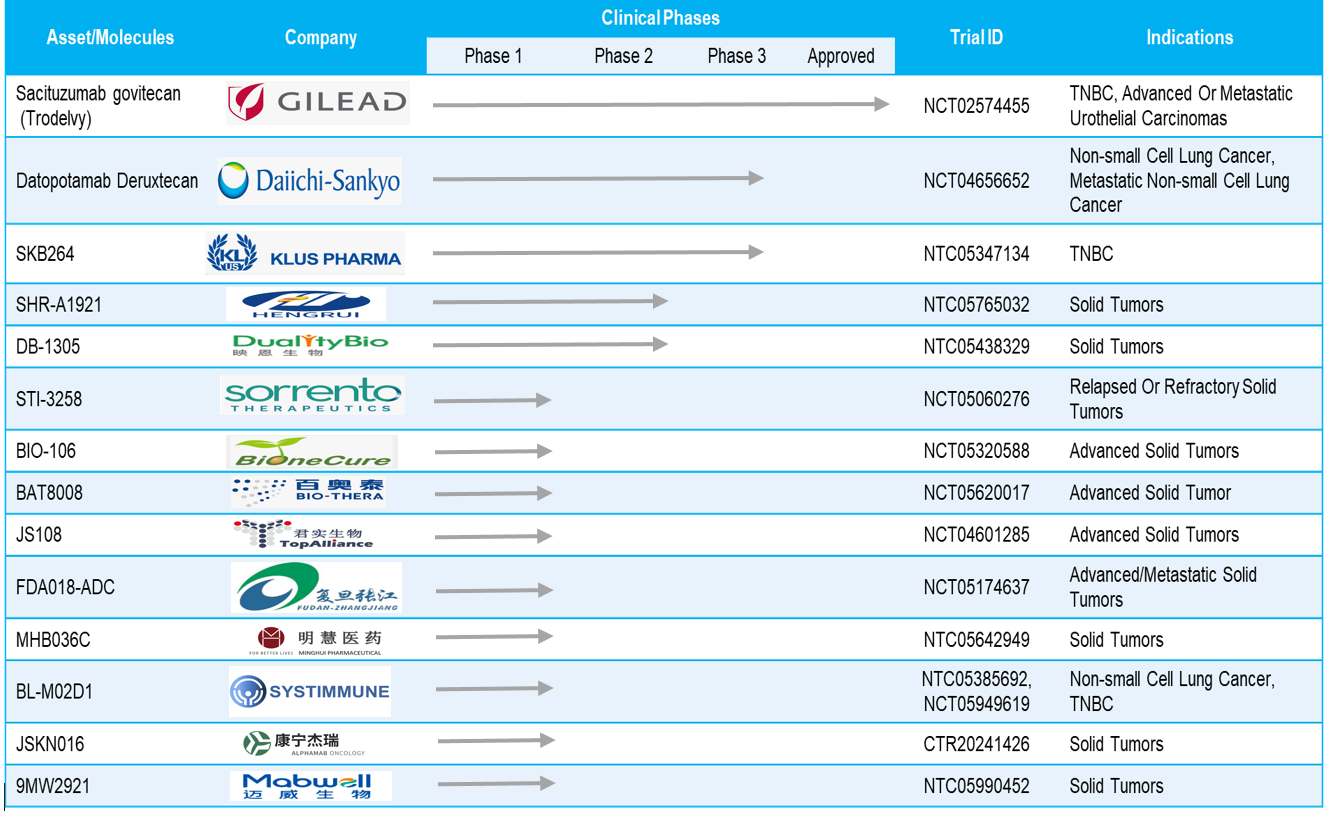
Table: Assets in Clinical Trials Targeting TROP-2
Key Players in TROP-2 Targeting ADC
Gilead, AstraZeneca, and Daiichi Sankyo are big pharmaceutical players in the Trop-2-targeting antibody-drug conjugates domain. Klus Pharma, the US subsidiary of China-based Kelun-Biotech, and Jiangsu Hengrui Pharmaceuticals are key Chinese players with assets in phase III and phase II clinical trials, respectively.
China is the leading country with nine Trop-2 targeting ADC assets in the pipeline, followed by the USA with four Trop-2 targeting ADCs (one approved and three in the pipeline) and Japan with only one Trop-2 targeting ADC asset in the pipeline.

Figure 5: Key players in TROP-2 Targeted ADC
Conclusion
We have observed a strong TROP-2 targeting ADC (anti-drug conjugates) clinical pipeline for the treatment of triple-negative breast cancer, non-small cell lung cancer, urothelial cancer, and solid tumors, making it instrumental in cancer therapy. Daiichi Sankyo and AstraZeneca are expecting regulatory approval for datopotamab deruxtecan (Dato-DXd) by the first quarter of 2025, and it would compete with Gilead’s Sacituzumab govitecan ADC therapy. Merck is also strengthening its ADC portfolio through a licensing agreement with Sichuan Kelun Pharmaceutical for its phase III asset SKB264. Further, Merck also collaborated with Daiichi Sankyo for proven ADC expertise and DXd technology to co-commercialize other ADCs such as patritumab deruxtecan, ifinatamab deruxtecan, and raludotatug deruxtecan worldwide. Two USA-based biopharmaceutical companies, Sorrento Therapeutics and SystImmune, are conducting phase 1 trials for their TROP-2 targeting ADC to treat solid tumors. More than nine small and medium-sized Chinese pharmaceutical companies have TROP-2 targeting ADC assets under the clinical pipeline. However, future research needs to focus on reducing ADC’s associated side effects and identifying biomarkers for tailored treatment.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.