Threat and Opportunity Assessment for Pharmaceutical Products
A pharmaceutical product is any drug product that is approved by the FDA or other county-specific regulators for the treatment of specific diseases after comprehensive research and clinical development to ensure its efficacy and safety in patients when administered at the recommended dose. Sales growth of marketed pharmaceutical products depends on multiple parameters, such as clinical efficacy being offered while treating patients and companies’ marketing and branding strategies across the geographies. The pharmaceutical industry is highly regulated by numerous regulatory bodies worldwide as drug products directly deal with patient’s life. Compliance with these regulations and guidelines is important to maintain a pharmaceutical company’s reputation and to avoid any legal consequences. These key threats may affect the pharmaceutical product sales growth during any phase of the product life cycle. Below are some key threats pharmaceutical products face during any phase of the product life cycle.
- Competitors/Generic Activity: Competitor activity on reference products decreases product sales due to price competition, which provides multiple options for patients—nearly patent expiry and loss of exclusivity lead to the risk of generic entry into the market.
- Loss of Patent Term & Exclusivity: After patent and exclusivity expiration, number of companies come up with generic versions of products which eventually decrease the sales of products. Pharmaceutical companies are facing major headwinds due to loss of exclusivity as product patents are near to expire, with billions of sales at stake for companies.
- Regulatory Barriers: The pharmaceutical industry is highly regulated by numerous regulatory bodies throughout the world as drug products directly deals with patient’s life. Companies may face different regulatory challenges in product approval, pricing approval, and GMP compliance as companies need to show compliance with these complex regulation and guidelines.
- Supply Chain Challenges: The supply of active and inactive ingredients that is being used in pharmaceutical products are mostly outsourced from multiple other vendors and chemical industries globally. For sustainable product growth, pharmaceutical companies need to manage the supply chain effectively and required long term plans on demand, supply, and production intelligently across the pharmaceutical supply chain.
- Infringement and Litigation Threats: Innovators may file litigation and infringement suits for innovative or branded products, which may causes monitory damages to pharmaceutical companies working on generic version of branded products.
- Price Regulation: Pharmaceutical industry are highly regulated in term of drug product pricing. The government polices may affect and regulate the prices of few products under specific categories to provide benefit the patients, which eventually reduce the revenue of certain products.
- Reimbursement Environment: Reimbursement policies of insurers may affect the sales and growth of some healthcare products.
- High R&D Cost: Drug development is associated with high R&D investment as it requires quality research and development facilities. So, research and development failure could be a big threat to innovative companies.
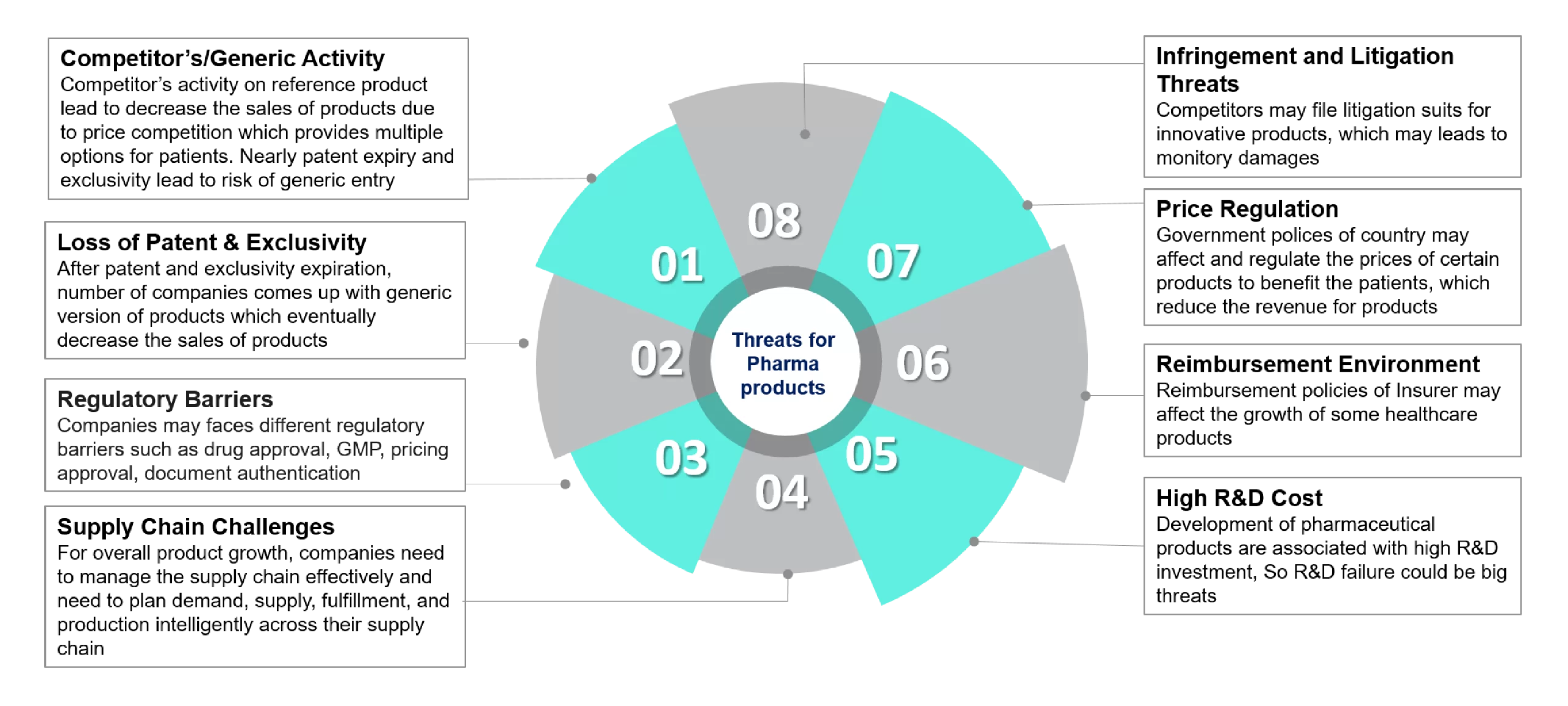
Fig 1: Threats for Pharmaceutical Products
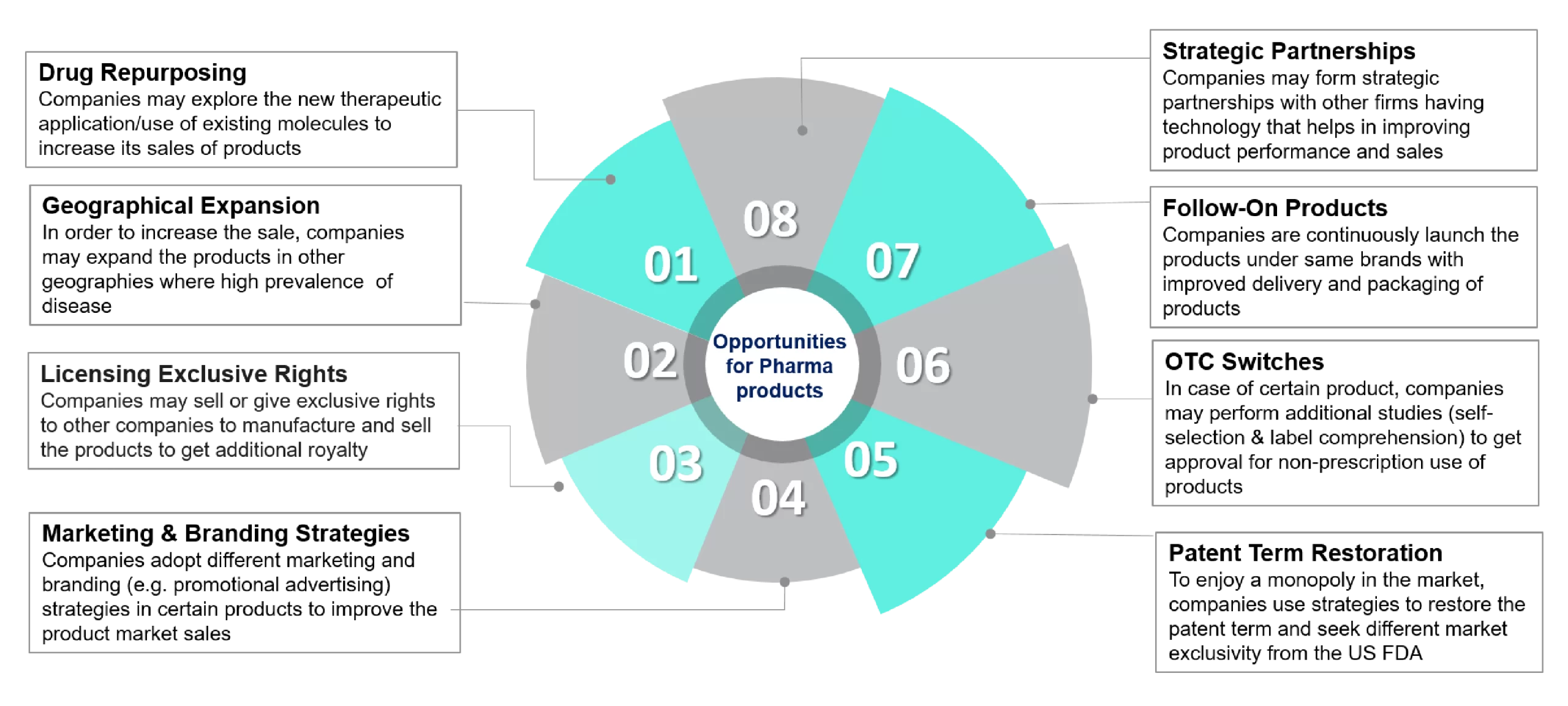
Opportunity for Pharmaceutical Products
In the process of drug development or after getting approval, pharmaceutical products may get specific opportunities to increase sales from approved pharmaceutical products by using fellow strategies for the products. Below are some key opportunities for pharmaceutical products:
- Drug Repurposing: In drug repurposing, companies can explore new therapeutic indications or therapeutic uses for approved existing molecules, which increases drug product sales. Multiple companies are using new technologies such as artificial intelligence and machine learning to identify novel therapeutic indications for already approved drug products.
- Geographical Expansion: To increase approved product sales, companies may expand their products to other geographies and regions or emerging markets with high prevalence and incidence of diseases.
- Exclusive Licensing Rights: Companies may sell or give exclusive rights for innovative medicine to other companies to manufacture and sell the medicine for additional royalties.
- OTC Switches: As a long-term strategy, pharmaceutical companies can perform additional studies, such as self-selection and label comprehension, on certain products to get approval for non-prescription use, which increases their market share and sales.
- Follow-On Product: Companies may launch products under the same brands with improved novel delivery systems, e.g., nanoparticles that improve drug absorption and packaging, to create a high-performing product portfolio under a single brand.
- Patent Term Restoration: To enjoy a monopoly in the market, companies use strategies to restore the patent term and seek different market exclusions, such as orphan drug exclusivity and pediatric exclusivity based on product types from the US FDA, to extend the monopoly in the market.
- Marketing & Branding Strategies: Companies adopt different marketing and branding (e.g., promotional advertising) strategies or enhance the distribution channels, both online and offline, for certain products to improve product penetration in the market and overall sales.
- Strategic Partnerships: Companies may form strategic partnerships with other technology companies or CDMO companies to enhance product benefits and, ultimately, sales. Strategic partnerships play a key role in the pharmaceutical industry and provide multiple benefits to pharmaceutical companies, such as increasing product market share, generating innovative medicine, and enhancing overall business performance.
Threats and Opportunity in Product Lifecycle
Drug discovery and development is a dynamic and complex process with significant threats and substantial opportunities for pharmaceutical companies. Each phase of the product lifecycle, such as (pre-launch, launch, and post-launch) possesses certain threats and opportunities for pharmaceutical companies.
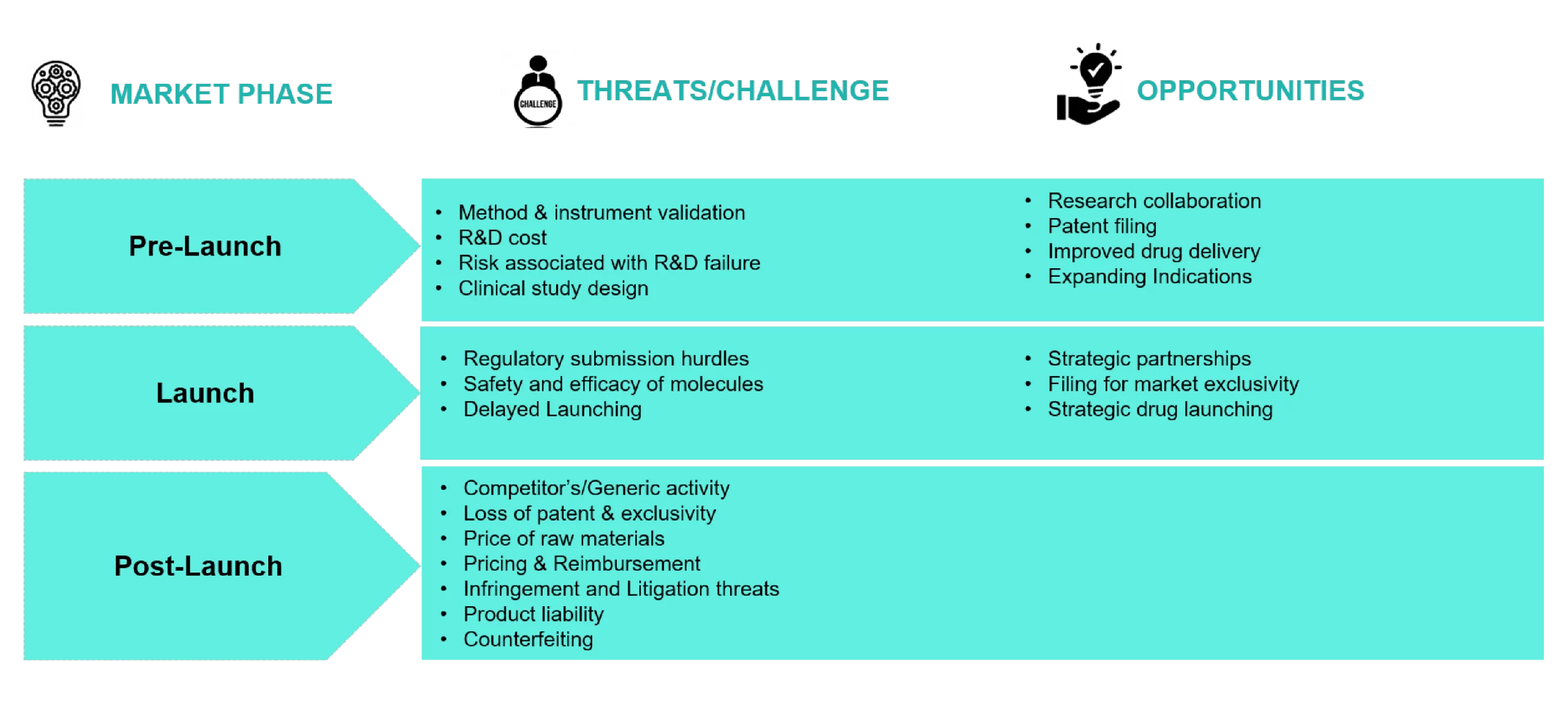
Fig 3: Threats and Opportunity in Product Lifecycle
Conclusion
Identification of potential threats such as regulatory changes, market competition, and supply chain challenges helps companies to create proactive strategies that will reduce the risks associated with drug products. The opportunity assessment studies by conducting competitive intelligence allow pharmaceutical companies to identify emerging trends and unmet needs. The pharmaceutical industry mainly uses SWOT analysis methodology to determine threats and opportunities for pharmaceutical drug products by considering multiple parameters associated with pharmaceutical products. The threat and opportunity assessment provides a solid framework for pharmaceutical companies to create innovative strategies to position companies in a competitive market by sustainably achieving their goals. Pharmaceutical industries can leverage insights and forecasting data from consulting firms to navigate threats and capitalize on emerging opportunities in pharmaceutical industries, ultimately enhancing competitiveness and product sales.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.