The Expanding Role Of Self-Amplifying RNA In Therapeutics
The approval of KOSTAIVE by Arcturus Therapeutics and CSL by the European Medicines Agency (EMA) marked the next chapter in the growing impact of mRNA vaccines worldwide. KOSTAIVE, however, is also a step ahead of the traditional mRNA vaccines. It is embedded with self-amplifying RNA (saRNA) that supersedes its standard counterpart in several ways. Though KOSTAIVE is not the first in this space, this approval signals the expanding realization of the vast potential of saRNA that goes beyond vaccines. A raft of applications, implications, and opportunities are waiting under its cover, and they will surely change the face of mRNA-based modalities in the near future. But first, let’s begin with the basics.
What Is saRNA, and How Is It Different from mRNA?
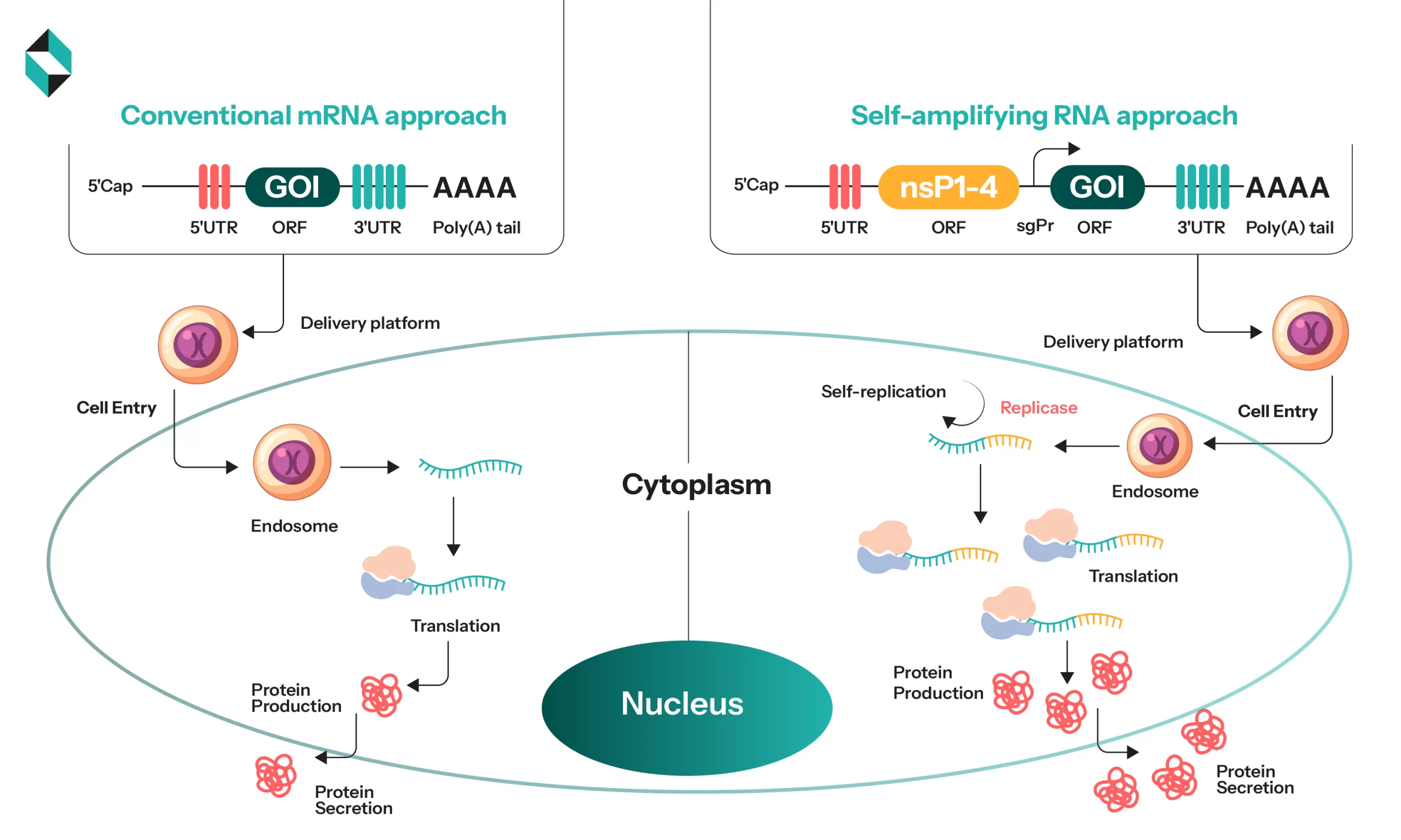
Self-amplifying RNA is identical to mRNA in its purpose and modus operandi, but with some structural differences and final results. Intentionally, it carries the genetic blueprint of the required protein for the target cell. But unlike normal mRNA, it has tools to self-replicate and amplify for a much stronger immune response.
Structurally, it is a much larger molecule with four extra non-structural proteins, the gene of interest (GOI), and a subgenomic promotor. The four extra proteins added are non-structural; they encode a replicase that amplifies the protein once it enters the target site.
The conventional mRNA systems are coded linearly, and they redirect the target cell’s ribosomes to create only a single protein. It is a time-consuming approach that requires multiple doses in higher quantities. In the saRNA, the replicase in non-structural proteins can amplify the original strand to a much larger size than its original self. Thus, they extend the duration and scope of protein generation for a single dose, thereby reducing the need for multiple doses.
Significant Advantages of saRNA Over Traditional mRNA
- Lower Injection Dosage: Due to its self-amplifying nature, saRNA vaccines need a much lower dosage to include an immune response equivalent to their traditional mRNA counterpart. For instance, only 5 μg/dose of saRNA vaccine ARCT-154 proved as efficient as Moderna’s 100 μg mRNA vaccine.
- Higher Efficiency of Antigen Production: Its ability to propagate within the host cell gives it the potential to achieve higher antigen expression levels in a single dose. This feature simplifies the vaccine administration process while reducing overall logistical challenges and the need for multiple injections.
- Enhanced Immune Response: During replication, saRNA can form double-stranded RNA structures. These structures closely resemble the viral RNA genomes identified by pattern recognition receptors (PRRs), which activate the innate immune response. This raises the immunogenicity of saRNA far above traditional mRNA vaccines, giving them an advantage for critical health conditions beyond infections.
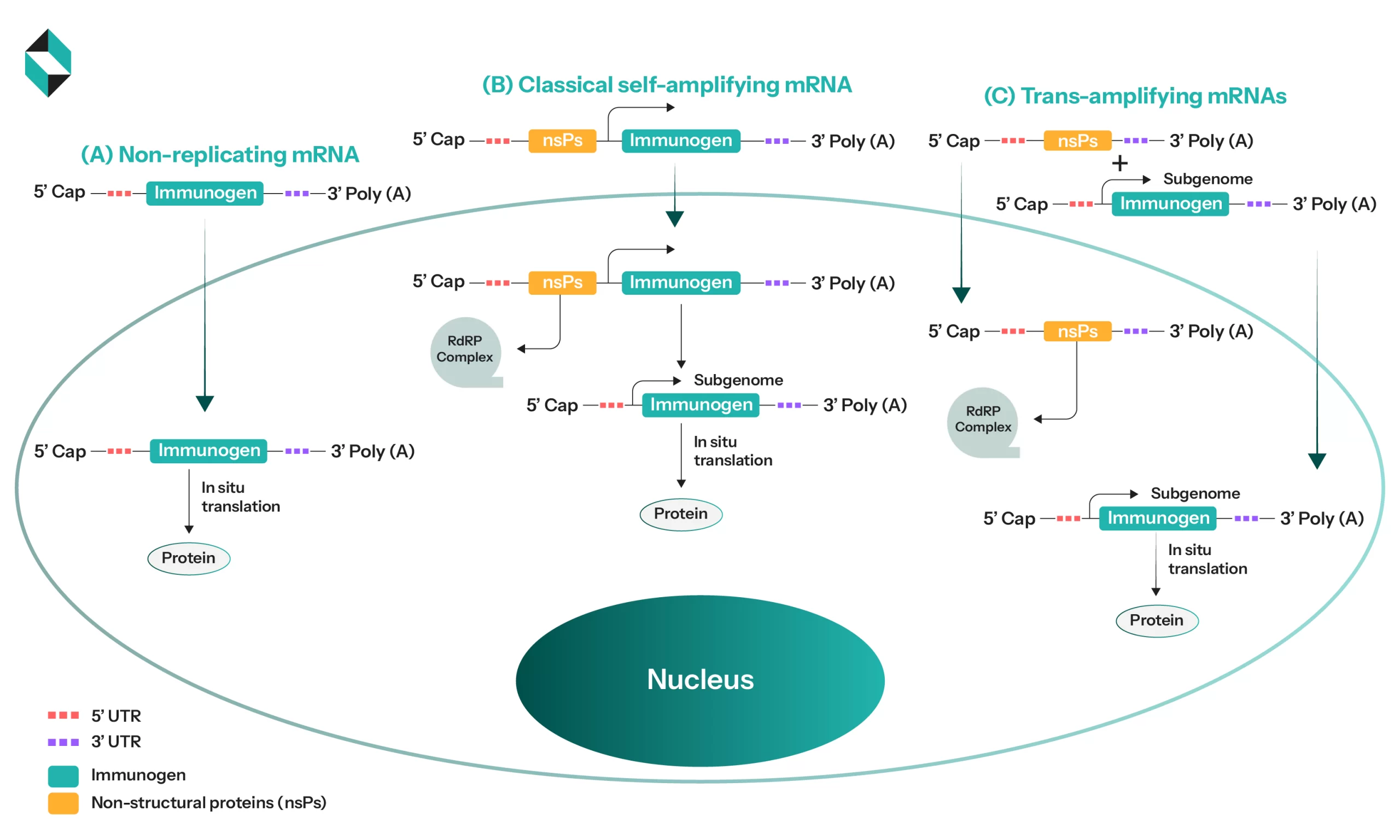
Schematic showing mammalian cell activity (A) Delivery of mRNA to cytoplasm (B) Delivery of classical saRNA to cytoplasm (C) Delivery of 2 different mRNAs for achieving equivalent immune response as classical saRNA (Source)
The saRNA Applications Beyond Vaccines
There is no second opinion on saRNA’s role in vaccine development. However, its transformative impact goes far beyond that. If combined and classified together, its application areas can be divided into three major classes:
- Vaccines: Besides COVID-19, saRNA vaccines are being explored for other infectious diseases, oncology, and proactive preparedness against plausible pandemics. For instance, in cancer treatment, saRNA can deliver genetic instructions to cells to produce cancer-combating proteins like immune-activating molecules, tumor-specific antigens, etc. Similarly, the enhanced stability, lower doses, and extended immune responses encouraged by saRNA have been characteristically helpful in responding to emerging viruses like influenza, COVID-19, and rabies.
- Therapeutic Applications: saRNA applications are also being explored in various therapeutic areas, including protein replacement therapy, ischemic stroke, encoding monoclonal antibodies, and treating chronic diseases like cystic fibrosis.
Schematic demonstrating the rationality of saRNA application in protein replacement therapy (Source)
- Ex-vivo Applications: In addition to in-vivo applications, saRNA has also proven its ability to modify cells ex-vivo. Therefore, its applications are being considered in several ex vivo processes, such as cell therapy and cell editing. Although broader RNA applications include tRNA for these purposes, they could complement saRNA applications in an ex vivo setting.
Market Players Pushing saRNA’s Horizons Beyond Vaccines
Arcturus Therapeutics: The company that recently received confirmation for its saRNA COVID vaccine for adults, KOSTAIVE, in Europe, has entered into a collaboration with CSL Sequirus to create advanced mRNA vaccines.
Keylicon Biosciences: A biotechnology company focused on innovative mRNA vaccines and on developing saRNA technology for infectious diseases and cancer treatment.
Replicate Bioscience: It develops saRNA constructs to prevent drug resistance in cancerous cells and treat other autoimmune diseases.
AstraZeneca: In association with the Imperial College of London, it is investigating the development of vaccines for respiratory diseases and a few others using saRNA technology.
Considerations for Manufacturers while Designing saRNA Workflow for Commercial Production:
- Purity: The purity of content must be prioritized, as it may compromise the regulatory compliance and therapeutic efficiency of the saRNA molecule. It is essential to check what impurities are critical according to the application. The most common impurities noted in saRNA production include dsRNA, protein residues, incomplete 5’ capping, poly A-tail heterogeneity, and topological variants of RNA. For instance, double-stranded RNA (dsRNA) produced during in vitro transcription can lead to harmful inflammatory responses in patients.
- Critical Process Parameters: To ensure the process’s efficiency, stability, and scalability, a detailed assessment of essential parameters needs to be optimized. The most recommended parameters include RNA sequencing and design, capping, transcription, and purification.
- Modified Nucleotides: Understanding the role of nucleotides in immunogenicity could be the key to mitigating innate immune responses unintentionally triggered by RNA molecules. Key industry players like Moderna and Pfizer used nucleotide modifications to improve the stability of their vaccines and it proved instrumental in their success.
- Capping Technologies: The right capping technology is pivotal in the stability and translation efficiency of saRNA molecules. As more continuous production platforms are integrated to produce GMP-compliant RNA within a day, the lessons learned could improve the production efficiency of saRNA molecules.
Final Thoughts
Despite its glory, saRNA faces several challenges in terms of molecule length, large size, and efficient delivery to target cells. Technologies to purify large-sized RNA molecules with around 4000-5000 bases are imminently needed. Its wide adoption is also limited by storage and transportation challenges, as poor stability of RNAs is an unsolved problem overall. The ongoing and near-future developments will assist saRNA developers in improving vector designs, manufacturing, and commercialization strategies.
How Can We Help?
The successful commercialization of saRNA promises an upper hand for biomanufacturers. However, to put this into practice, a pragmatic approach and strategy need to be built that reduces time spent on marketing and streamlines the process. An expert’s counsel in core production areas like RNA capping technologies, nucleotide modification, and analytics could be pivotal in addressing these challenges. That’s where we come in. Our life sciences and biotechnology experts have helped several clients with horizon scanning, partner scouting, and business and market strategy development in the last 15 years. We are determined to simplify the complications of mRNA, gene editing, and other biotechnology spaces. To understand better, reach out to us.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.