Bispecific Antibody-Drug Conjugates: Clinical Development and Future Directions
In the last decade, ADCs have been an emerging class of medical modalities to treat cancer by combining potent anti-cancer medicines (i.e., payloads) with monoclonal antibodies that deliver cancer medicines to the target cells. According to the market reports, the global ADC request size was valued at around 11.84 billion USD in 2024 and is projected to reach USD 12.89 billion in 2025 and USD 25.38 billion by 2033, growing at a CAGR of 8.84% during the period (2025- 2033).
ADCs include three important factors: monoclonal antibodies, linkers, and anti-cancer medicines. ADCs can deliver anti-cancer medicines directly to the inside of target cells so they do not damage the near-healthy cells. Biopharmaceutical companies have been exploring bispecific ADCs, ADCs with site-specific conjugation, and dual payload ADCs. These linkers have essential places in the pharmacokinetics of ADC and the overall success of the ADC.
Several well-established anticancer medicines, such as DNA topoisomerase and microtubule inhibitors, are used as payloads in developing ADCs. Bispecific antibodies have been a crucial trend in the ADC space as they enhance target specificity and provide diversity to treat cancer. Bispecific antibodies can target two epitopes of a target protein or two different targets at the same time, therefore producing better efficacy through multiple different molecular mechanisms. This approach is particularly promising for treating cancer with an expression of multiple target proteins, wherein multiple pathways frequently drive the progression.
However, the design of bispecific ADCs requires extensive immunological understanding to tackle the challenges associated with the light and heavy chain mismatches, and a lack of thermal stability at manufacturing can lead to the failure of bispecific antibodies. The bispecific ADCs currently under investigation have been categorized into the following two categories:
- Bispecific ADCs targeting dual tumor-associated antigens (TAA) (e.g., MUC1/EGFR-ADC)
- Bispecific ADCs targeting dual epitopes (e.g., HER2/HER2-ADC).
Advantages of Bispecific Antibody Drug Conjugates (ADCs)
Bispecific ADCs have the following key advantages over conventional single-target ADCs:
- Targeting 2 spots can enhance specificity and reduce the toxicity to normal cells.
- The ADCs can produce better internalization and enhance the killing effect.
- Bispecific ADCs can overcome the problem of medicine resistance caused by the dropped single-target expression.
Get the latest insights on bispecific ADCs—clinical progress and future directions; download the full article now.
Bispecific ADCs: Clinical Development
So far, around ten active bispecific antibody-drug conjugates are under clinical development. Below are some highlights on bispecific antibody-drug conjugates that are under clinical development.
- M1231: SUTRO developed M1231, an EGFR/MUC1 bispecific antibody-drug conjugate. It uses the company’s proprietary fixed-point coupling technology to attach Hemiasterlin derivatives to specific amino acid sites of the antibody via a cleavable linker (DAR—4). Sutro collaborated with Merck KGaA to develop M1231.
- Zanidatamab Zovodotin (ZW-49): ZW-49 is a bispecific ADC under phase 1 clinical trials developed by Zymeworks that specifically binds two epitopes of the HER2 receptor. The bispecific ADC molecule is based on ZW25 coupled to Auristatin toxin A through a protease cleavage linker.
- REGN5093- M114: REGN5093- M114 is a bispecific ADC developed by Regeneron that targets two different epitopes of MET. The antibody of REGN5093- M114 is an asymmetric bispecific antibody that links to the maytansine derivative via an M114 linker (DAR: 3.2).
- Zalontamab Brengitecan (BL-B01D1): BL-B01D1 is an EGFR/HER3 bispecific ADC developed by SystImmune in collaboration with BMS, which adopts its unique AC linker to link the camptothecin derivative ED04 to the cysteine site of the antibody (DAR-7.5).
- JSKN003: Alphamab Oncology developed JSKN003, a HER2-targeting bispecific antibody-drug conjugate developed in-house using a proprietary Glycan-specific conjugation platform. JSKN003 presents excellent, encouraging preliminary antitumor activity in heavily pretreated patients with advanced solid tumors.
- TQB 2102: Chia Tai Tianqing Pharmaceutical developed TQB 2102, a bispecific ADC that targets two non-overlapping epitopes of HER2, ECD2, and ECD4 to treat HER2-positive cancer.
- AZD9592: AstraZeneca developed AZD9592, a bispecific Ab targeting EGFR and c-MET with a topoisomerase 1 inhibitor (TOP1i) as a payload for treating solid tumors.
- EBC-129: The National Cancer Centre Singapore developed EBC-129, the first made-in-Singapore antibody-drug conjugate. EBC-129 binds to CEACAM5 and CEACAM6, which are specific glycosylation sites of carcinoembryonic antigen-related cell adhesion molecules – CEACAM5 and CEACAM6.
- IMGN151: IMGN 151 is an antibody-drug conjugate (ADC) targeting the folate receptor alpha (FRα) being developed by ImmunoGen, a subsidiary of AbbVie, for the treatment of endometrial and lung cancer.
- KM-501: Xuanzhu Biopharma is developing a bispecific antibody-drug conjugate targeting human epidermal growth factor receptor 2 (HER2).
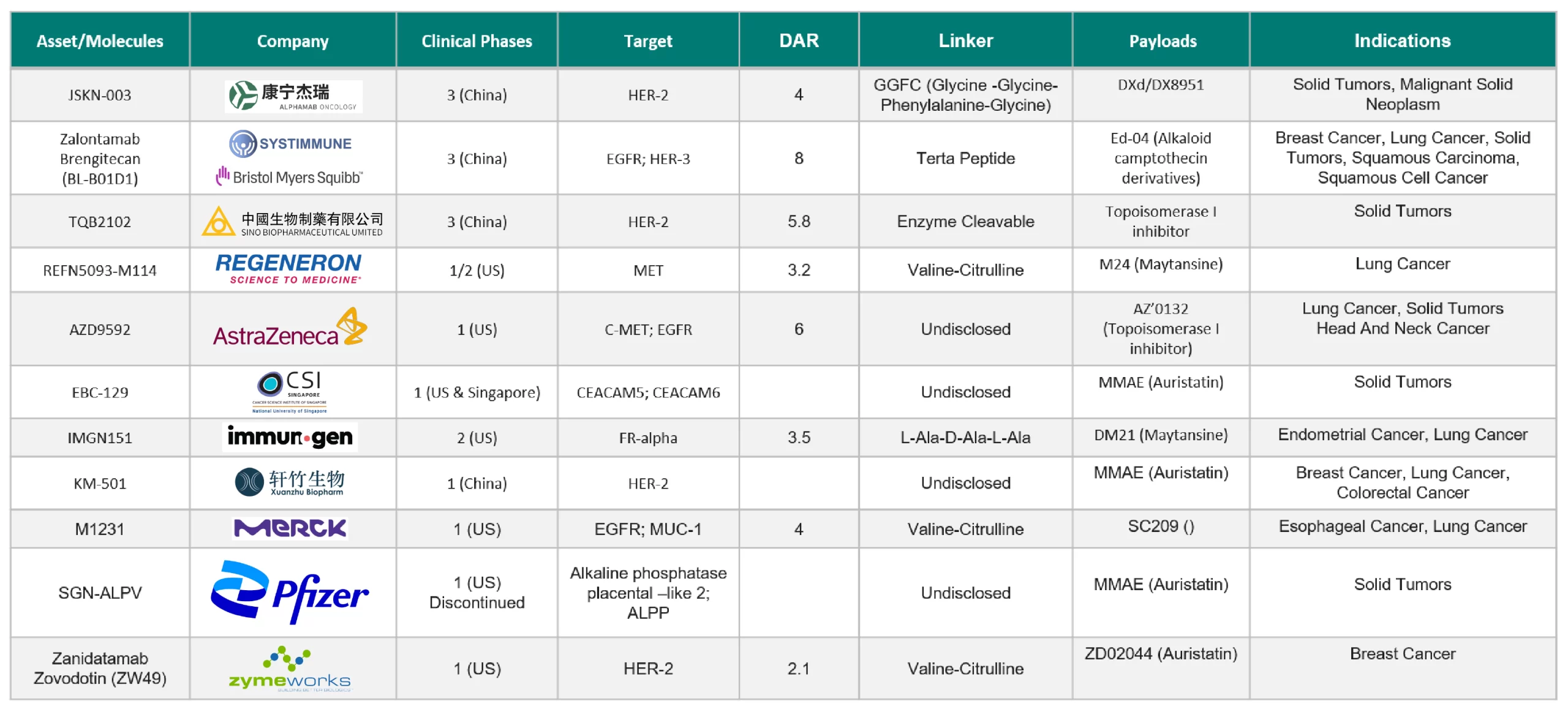
Table 1. Clinical Development Pipeline of Bispecific ADCs
Key Players in Bispecific ADCs
Pfizer, AstraZeneca, and Merck are big pharmaceutical companies working on bispecific ADCs to enhance tumor specificity and address cancer heterogeneity. Other key players include Alphamab Oncology, Zymeworks, Regeneron, Sino Biopharmaceuticals, and SystImmune, which work on the clinical development of bispecific ADCs. The USA and China are the leading countries with the maximum bispecific ADCs in the clinical pipeline, followed by Canada and Singapore, which have single bispecific ADCs under clinical development.

Future Directions
Developing bispecific ADCs is a more complex process than developing conventional monoclonal antibodies and requires designing antibodies with different binding specificities and affinities. Developing bispecific ADCs without affecting the stability of the bispecific antibody remains a significant challenge. Although BsADCs are designed to target tumor-specific antigens or proteins, sometimes they interact with non-tumor tissues that share similar antigens and produce cross-reactivity that creates toxicity and side effects. Designing the pharmacokinetic parameters of bispecific antibodies is more complex than that of traditional monoclonal antibodies due to their specificity to multiple targets, which can affect the distribution and effectiveness of the conjugate.
Recent advanced technology can be used to understand the tumor heterogeneity in various cancers to refine target structure and affinity, enabling the development of BsADCs. Companies and research institutes are addressing the issues related to the complexity of ADC design using machine learning and artificial intelligence. Further, companies and research institutes are working on target specificity, immunogenicity, tumor resistance, and pharmacokinetics to unlock the development of advanced bispecific ADCs with full therapeutic potential and reduced side effects that could revolutionize cancer treatment.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.