Automation in Bioprocessing
Bioprocessing involves culturing microbes or living cells to produce essential and valuable substances, such as pharmaceutical compounds. In the field of biopharmaceutical manufacturing, it is imperative that these bioprocesses are optimized for sustainable and innovative solutions. Integrating automation in these processes will increase efficiency, reduce human errors, and improve accuracy.
Bioprocess automation is governed by the use of sensors, actuators, and controllers to monitor important production process parameters such as temperature, pressure, pH, and dissolved oxygen. It also involves real-time monitoring of samples during various stages of bio manufacturing: cell culture and cell line development, upstream processing, downstream processing, and fill and finish.
Advantages of Automated Bioprocessing
Automation offers great advantages to biopharmaceutical manufacturing, resulting in high yields of products with excellent quality:
- Optimized Process Control: Automation allows process control and real-time monitoring of the manufacturing workflow. This improves the product quality and reduces the risk of deviations among the different batches.
- Enhanced Product Quality: Automation enhances the quality of the produced biopharmaceuticals by reducing operator interaction and simplifying the process. It also allows for early detection of process deviations and further taking necessary corrective measures in time.
- Dealing with Variability: The automated process provides optimal process conditions and minimizes the effect of unit operation error, thereby reducing variability across various batches.
- Enhanced Throughput and Efficiency: Automation would allow for continuous bio manufacturing by reducing the time required for manual tasks. For instance, integration of downstream processing equipment, such as chromatography and filtration, can be integrated into an automated workflow, which will reduce time.
- Scalability: It is a prime requirement as the production has to be taken from the lab to a large scale. Automation will assist in optimizing and adapting these processes at a large scale.
- Sustainability with Single-use Bioprocessing: The environmental impact of the bioprocesses can be greatly reduced by utilizing the automated single-use bioprocessing, as they do not require an extensive cleaning process. In addition to this, exploring various waste management options will further reduce the ecological impact of the disposable materials.
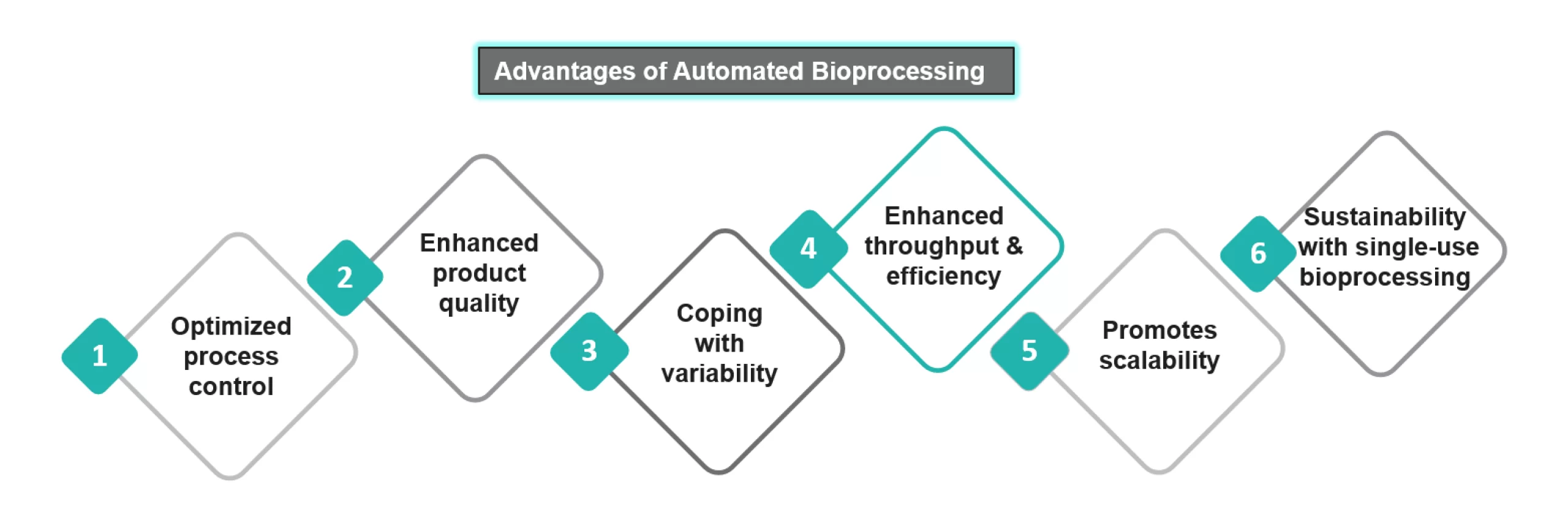
Figure 1: Advantages Offered by Automation in Bioprocessing
Artificial Intelligence Technologies in Bioprocess
Artificial intelligence can be applied to optimize the critical process parameters (CPPs) in bioprocessing for process control and optimization:
- ML algorithms have been used to understand the relationship between various variables in the process.
- An artificial neural network (ANN) was used to model and optimize the production of a biopolymer through fermentation
- Optimization and process control of hyaluronic acid production have been explored by Radial basis function neural work (RBF-ANN) and particle swarm optimization (PSO).
- Deep learning approaches have been used to monitor the critical process parameters (CPPs) according to online sensor data in anaerobic digestion (AD).
- A hybrid multi-objective strategy was applied to simultaneously optimize the biomass and production yield of microorganisms for industrial production.
Market Overview
The bioprocess automation market size has seen a remarkable growth in recent years owing to several factors, such as an increase in efficiency, a reduction in cost, increasing demand for remote monitoring and controlled systems, and limiting labour dependency. It is further expected to grow rapidly in the coming years due to the integration of AI and machine learning, digitalization, process control strategies in gene and cell therapy, data analytics, and visualization. The advent of personalized medicine in the years ahead will fuel the bioprocess automation market in the near future. The automated bioprocess systems will enable precision and scalability in manufacturing patient-specific drugs.
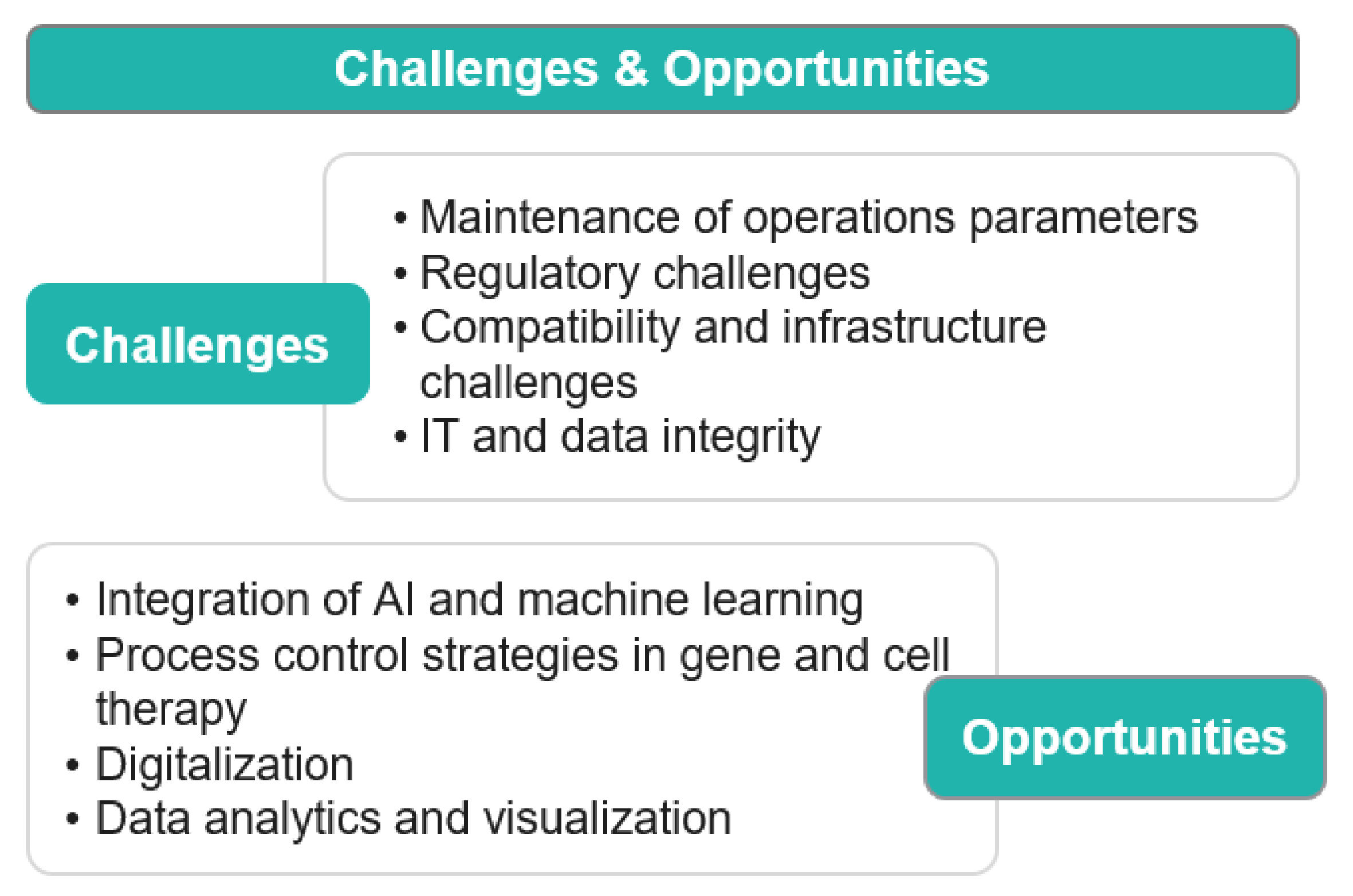
Figure 2. Market Overview
Key Players and Their Recent Activities
- Cytiva has recently launched its X-platform bioreactors to ease the single-use upstream bioprocessing operations. Due to its ease of operation, flexibility, and enhanced efficacy, it can be used to produce cell and gene therapies, antibodies, and viral vectors.
- An integrated bioreactor system has been collectively launched by Sartorius and Repligen Corporation, wherein the Sartorius Biostat STR® bioreactor, along with Repligen XCell® ATF upstream intensification technology, will simplify the seed train and N perfusion implementation in biomanufacturing.
- Cytiva and Culture Biosciences are exploring innovation in upstream bioprocessing together, with an aim to enable scalability (June 2023).

Figure 3: Key Players Working in the Domain
Conclusion
Automation is beneficial to the biopharmaceutical manufacturing process due to its ability to improve process control, particularly in providing real-time monitoring of critical process parameters. It will prove to be a catalyst for change in the industry by converging the scalability of bioprocesses from laboratory settings to large-scale commercial production. Further to this, the adaptation of machine learning with bioprocessing will allow biotechnology companies to revolutionize process control to accelerate experiment planning and decision making.
With numerous advantages in hand, automation is expected to dominate the bioprocessing industry in the upcoming years, where precision, reproducibility, and scalability will no longer be distant goals but achievable targets.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.