
Pharmaceuticals
Enabling innovative medicines for global health
Building A Healthier Future
Along with Artificial Intelligence and digital therapeutics integration, blockchain technology inclusion is a must for secure supply chain monitoring at all ends. Pharmaceutical companies must strategize better to combat supply chain disruptions, economic uncertainty, geopolitical pressures, and pandemic-triggered R&D gaps. As our pharmaceutical consulting team puts it a “zero trust” approach is mandatory to resolve the gaps and ensure that the cyber vulnerability of all suppliers is neutralized. Industry participants also need to take a stand on the “China plus one” concept while addressing the impact of climate change on global supply chains.
The pharmaceutical industry, traditionally slow to adopt new technologies, is experiencing rapid transformation. Various key advances such as Artificial intelligence (AI) and automation, advancement in conducting clinical trials, sustainability, green initiatives, and digital technologies, etc., will have a major impact on the pharmaceutical industry over the next 5-10 years. The small molecules will continue to rule the pharmaceutical market, too. However, the usage and popularity of biologics are also expected to grow faster in the near future. Gradually, more innovative and effective drugs will be launched over the next few years.
Personalized medicine is on the rise, recognizing individual treatment needs. Pharmaceutical companies will adopt new supply chain software technologies to improve resilience and competitiveness. Artificial intelligence (AI) and automation are enhancing efficiency in analyzing data and drug development processes. Despite industry complexity, the future looks promising with ongoing technological advancements and a commitment to innovation.
Industry Trends
Pharmaceuticals Trends
Real World Data (RWD) Integration
Decentralized Clinical Trials (DCT)
Sustainability and Green Initiatives
Digital Therapeutics
Supply Chain Resilience
Real World Data (RWD) Integration
The integration of Real World Data (RWD) in pharmaceuticals is picking up pace very rapidly, driven by the increasing adoption to enrich drug development, clinical trials, and regulatory approvals. It is expected to grow to USD 3.8 billion by 2028 at a CAGR of 18 percent. RWD, from patient records, wearables, and health databases, is a trend allowing businesses to optimize drug efficacy, improve safety monitoring, and accelerate market access. This comprises huge growth prospects for investors with increased demand for data-driven insights across the pharmaceutical sector. Companies that put RWD at the front of innovation, cost reduction, and personalized treatments are indeed set to shape an increasingly evidence-based healthcare landscape in pharmaceutcials consulting experts’opinion.
How Can We Help You?
Strategic foresight methodologies are widely used in the life sciences industry to identify medium to long-term risks and opportunities. The process involves assessment and identification of emerging technologies and gaps at an early stage. It is a way to scan, identify, and stay up-to-date on critical current and emerging technologies. Horizon scanning helps to understand potential business impact, benefits, costs, adoption challenges, risks, and technology debt. It also helps to understand the security factors, skills, and implementation considerations of potentially critical technologies and broader implications. Identifying opportunities and potential risks can be difficult amidst prevalent market noise and hectic day-to-day work schedules. Strategic foresight can be a great way to do this.
ExploreLife sciences companies and healthcare providers have lately faced large-scale economic pressure and significant regulatory changes. Keeping up a steady stream of cutting-edge products and services is essential for the expansion of these companies. Stellarix’s life sciences consulting team excels in developing research and development (R&D) plans that maximize and improve every phase of the product life cycle in collaboration with industry leaders across all therapeutic areas. From business development planning to bolstering the innovation portfolio, insights on cutting-edge technologies and evidence-generation tactics, our professionals assist in laying the groundwork to remain relevant against evolving industry disruptions. Through a combination of conventional and advanced techniques, such as portfolio optimization and process modifications, we assist life sciences firms redirect their R&D efforts to stay ahead of the curve.
ExploreHealthcare companies are making efforts to reimagine value chains and business models in the wake of rapidly changing, digitalizing, and transforming industry dynamics. As a marketing strategy is a subset of business strategy that focuses on the marketing initiatives, the business strategy itself refers to the long-term plan of action that many organizations use to achieve their goals and objectives. It is measured in terms of overall business performance indicators, such as revenue, profit, and market share, and terms of marketing-specific indicators. To help organizations achieve these milestones, our healthcare consulting team offers proper market analysis, target audience identification, product portfolio optimization, continuous monitoring, and evaluation services.
ExploreAchieving sustainability in the cost-and quality-sensitive life sciences industry is difficult but not impossible. To address environmental, social, and governance (ESG) considerations and foster long-term success, reliable development of sustainability strategy is required. Sustainable R&D, supply chain sustainability, innovation for sustainability, eco-friendly approaches, lifecycle assessments, etc., are a few aspects supporting the formulation of robust sustainability strategies in the dynamic world of healthcare. Several life science companies are working toward sustainable development in their respective fields. Reducing waste and recycling, developing greener architectural designs, choosing less toxic alternatives, managing energy resources efficiently, and supply chain optimization are some of the ways through which sustainability is being integrated into this sector.
ExplorePost-pandemic, life sciences companies are looking to improve device interoperability, outcomes, and actionable insights execution without losing their edge in the intensifying market competition. The requisites presented by these changes raise the need for co-man and partner identification to facilitate collective recognition of unmet needs and feasibility validation of new ideas. A collaborative approach can lead to the development of technological empowerment, making new initiatives and efforts more robust, user-friendly, and clinically relevant. When organizations collaborate and form partnerships, their full potential is realized as they work together to achieve common goals and exchange expertise. Collaborations and partnerships in the life sciences industry involve different organizations, such as hospitals, technology manufacturers, government agencies, non-profit organizations, pharmaceutical companies, biotech firms, research institutions, academic centers, etc.
ExploreStartup strategy is a business plan that outlines how an organization plans to accomplish its objectives. There are a few key elements for a successful startup strategy in the life sciences industry that span goals, resources, research, analysis, scientific foundation, regulatory status, IP protection, competitiveness, financials and funding strategies. It is easy to understand the complex landscape of scientific, regulatory, and business challenges with strategic startup strategy. We assist in developing strategy through market research, regulatory compliance, robust product development, and intellectual property protection. Our approach includes clinical validation, strategic partnerships, and innovative business models accommodating changing industry dynamics and standards.
ExploreOur Experience
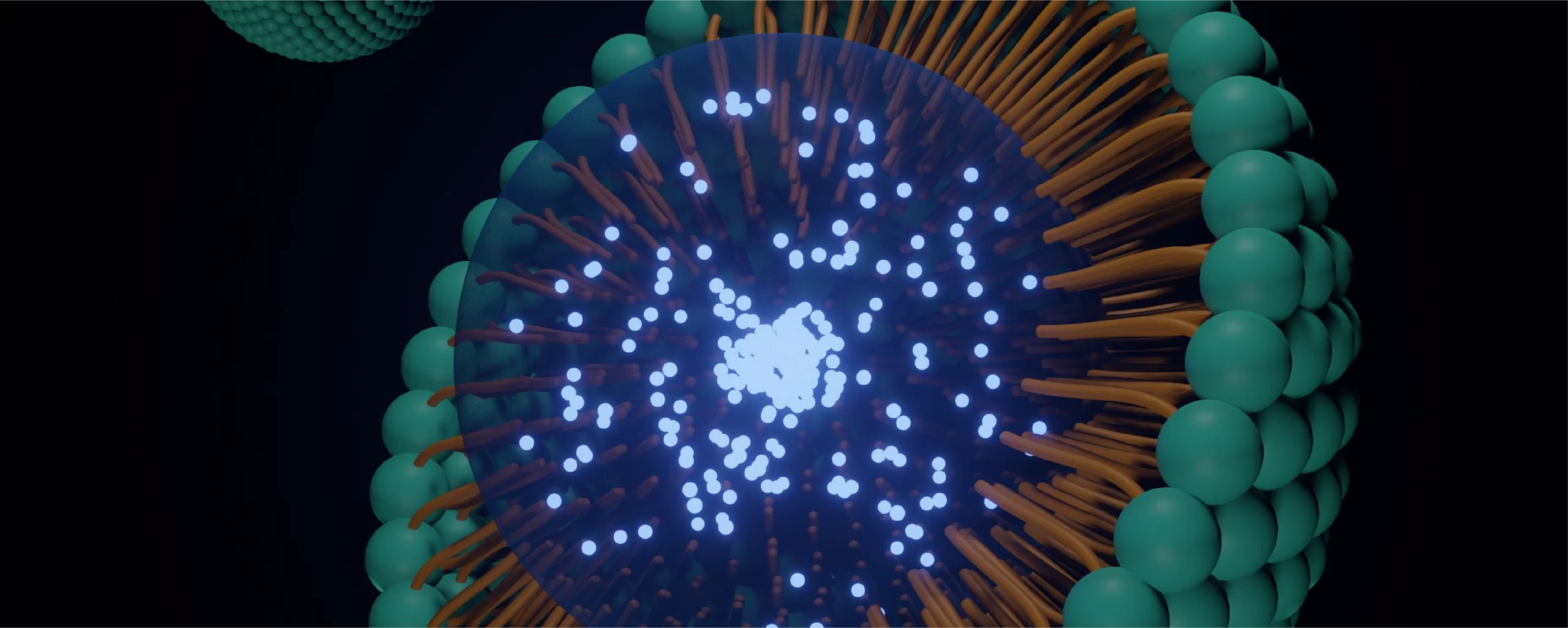
IP Landscape- Lipid Nanoparticles for Biomolecules Delivery
A global pharmaceutical leader partnered with Stellarix to gain strategic insights into lipid nanoparticle (LNP) systems for biomolecule delivery. With LNPs emerging as a critical platform for mRNA, siRNA, and CRISPR-based therapeutics, recognized and regulated by the FDA—our case study mapped key components, formulation strategies, and advanced manufacturing practices. Client Background: The client is a […]

Landscape: Child Resistant Packaging
A startup engaged with Stellarix to learn more about the child-resistant (CR) packaging market, technological advancement, size, trends, and regulations of the landscape. It requested that the CR packaging segment be explored, as well as emerging innovations and major players participating in the sector. The study provided the client with important insights that helped the […]
Technology Scouting: Emerging Technologies for Drug Traceability
A top pharmaceutical company contacted Stellarix to learn more about technological advancements like smart sensors and artificial intelligence in the industry to enhance drug traceability. The client wanted to delve into regulatory compliance, reliability, and pharma tracking and grasp an edge over competitors with accuracy and operational efficiencies, offering cost-effectiveness, safety, and leadership. Client Background […]
Client Queries Addressed
Q1. Which therapeutic areas (e.g., oncology, cardiovascular, rare diseases) are most impacted by Personalized Medicine? Q2. What are the emerging technologies (e.g., genomics, AI-driven diagnostics) that are driving Personalized Medicine advancements? Q3. How are regulatory environments evolving globally to support Personalized Medicine initiatives?
Q1. Which AI-driven technologies (e.g., machine learning, natural language processing) are showing the greatest impact in drug discovery? Q2. How are AI platforms being used to predict drug-target interactions and identify potential therapeutic candidates? Q3. What partnerships or collaborations exist between pharmaceutical companies and AI technology providers to enhance drug discovery processes?
Q1. What are the leading GenAI platforms currently being used for de novo drug design? Q2. How can GenAI accelerate the identification and synthesis of novel drug candidates? Q3. What partnerships exist between pharmaceutical companies and GenAI providers to enhance drug discovery processes?
Q1. How can pharmaceutical companies ensure consistent and reliable data collection across decentralized trial sites? Q2. What technologies and platforms are available to support real-time monitoring and data integration in DCTs? Q3. How can partnerships with CROs and technology providers enhance data management and compliance in DCTs?
Insights
See All
Articles

Articles

Blogs

Blogs