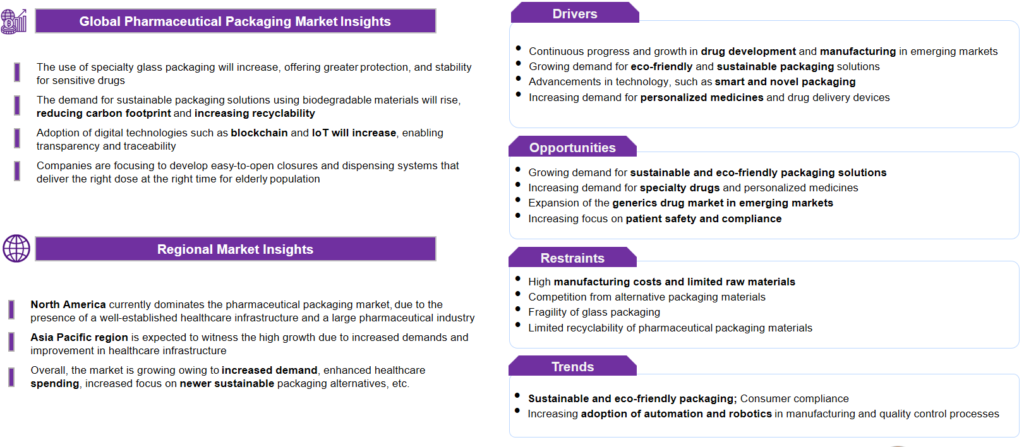
In today’s ever-evolving landscape, pharmaceutical packaging transcends traditional safety and integrity measures, incorporating state-of-the-art technology to enhance patient adherence and advocate for sustainability. This represents the vanguard of pharmaceutical packaging systems, where protection is merely the foundation.
Pharmaceutical packaging systems are vital for protecting drugs against external harmful factors. They warrant appropriate dosage, maintain the proper condition of the medicine, and specify necessary information for patients and healthcare professionals.
There are three main types of pharmaceutical packaging: primary, secondary, and tertiary.
a). Primary packaging (E.g., blisters, ampoules, vials, sachets, etc.) is in direct contact with drugs and medicines.
b). Secondary packaging is another layer of packaging that holds the primary packaging (E.g., cartons, boxes, etc.).
c). Tertiary packaging is important for the shipping process. It safeguards primary and secondary packaging (E.g., barrel, Edge protector, etc.).
Adoption of QR Codes for Packaging
- QR codes on packaging reduce the need for physical pamphlets or leaflets for drug product
- Various regulations have been imposed in the US, Europe necessitating pharma companies to attach QR codes on medicine packages
Use of Smart Glass Technology
- Smart glass technology, which can change its properties in response to external stimuli such as light, heat, or electricity, is being explored for use in drug delivery systems
Increased Focus on Sustainability
- Companies are exploring ways to reduce the environmental impact of drug containers. Bio-pharma companies collaborating for sustainable packaging (E.g., Sanofi joined PulPac for fiber-based blister pack)
Smart and Connected Packaging
- Smart and connected packaging is a growing trend as digital technologies have become more efficient. Companies leveraging this to improve adherence, track medications, and ensure the authenticity and safety of their products
- Use of biodegradable, recyclable, and compostable materials such as corn starch, mushroom, and biomass-based plastic from plant-derived materials
- Printing product and patient information directly onto the secondary packaging can reduce the waste of unnecessary individual labels and safety leaflets
- By adopting a Quality by Design (QbD) approach, manufacturers can minimize waste, degradation, and contamination from substandard or poorly designed packaging
- The rise of pre–filled pharmaceuticals can reduce the environmental impact and the risk of patients having dosage errors
| CHALLENGES | SOLUTIONS |
| Protecting the pharmaceutical product during handling, storage, and transport | Using rectangular boxes for packaging, as handling of rectangular boxes is easy During transport, use strong, good quality, and resistant packaging materials, along with the proper maintenance of temperature and humidity conditions |
| Counterfeit products are a major issue | Pharmaceutical companies are now incorporating scannable codes, stickers, UV prints, tear tape seals, etc., to ensure that the genuineness of products is preserved |
| Environmental and sustainability issues | Incorporate eco–friendly materials and designs for packaging to minimize carbon emissions. The circular economy is one such solution. |
| Adhering to regulatory and global standards | Make the labeling and printing universally accepted to the best possible extent Following the ICH pharmaceutical regulations is the best way to have a wide and standardized framework for packaging, in addition to GMP and ISO standards |
| Packaging for new medicines | Develop new and unique types of packaging that are inserts and can be easily used for newly developed medicines. Quality by design (QbD) principle can be followed in such cases |
The pharmaceutical packaging market is comprised of numerous major players such as Amcor, Gerresheimer, BD, Schott, Stevanato, West Pharma, etc., and many emerging start-up companies such as Comar, Ecobliss, ALPLA pharma, Catalent, etc. Pharmaceutical packaging companies contribute considerably to the market’s growth by providing more protection, patient-friendly designs, child-resistant packaging, eco-friendly products, personalized solutions, etc.

Figure: Various Companies in the Pharmaceutical Packaging System Domain
Multiple collaborations and strategic partnerships have been seen recently. West Pharmaceutical expanded its collaboration with Corning to bring sustainable and performative drug delivery solutions to market. Gerresheimer AG and Stevanato Group announced a collaboration to develop an innovative ready-to-use vial platform for the pharmaceutical industry. Recently, Amcor acquired Phoenix Flexibles, a scalable, flexible packaging company.
These activities will lead to more innovation and advancements in the pharmaceutical packaging domain. With new opportunities and developments, multiple challenges need to be addressed to drive innovation, address unmet needs, and improve patient’s quality of life.
The attributing factors for market growth include, but are not limited to, the growing population, increasing health awareness, sustainability measures, and growing demands. Moreover, growing awareness of eco-friendly, biodegradable products and the adoption of new regulatory standards for recycling are some of the key driving factors.