de novo Biologics’ Freightage: Exosomes
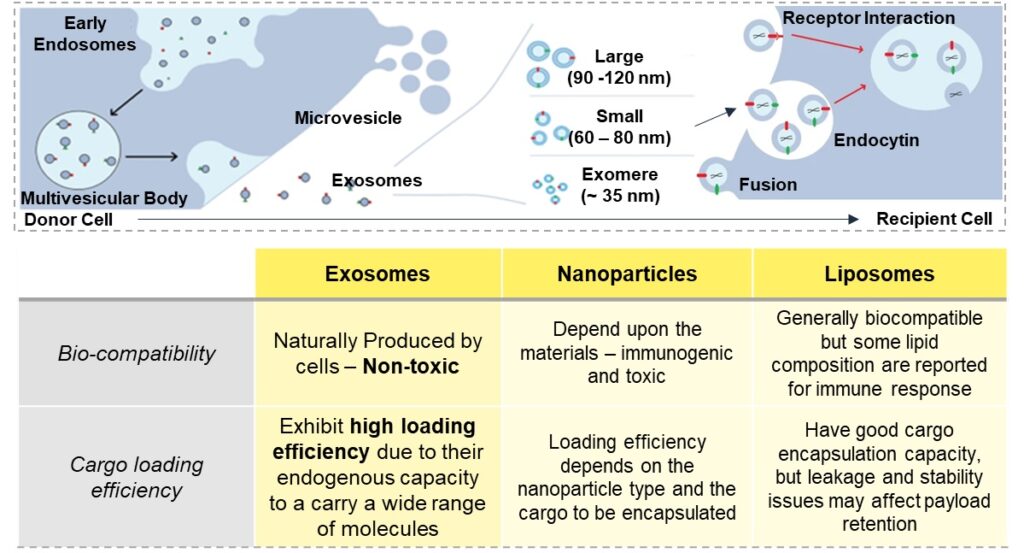
Exosomes are tiny biological vesicles secreted by cells and hold immense promise in the field of therapeutics. Unlike traditional nanoparticles, exosomes offer a natural and safer avenue for drug delivery due to their inherent biocompatibility. These are natural carriers with low toxicity and generate low immune response. Moreover, exosomes boast exceptional cargo-loading capabilities, allowing for the efficient transport of various therapeutic agents, including hydrophilic and hydrophobic drugs, DNA, RNA, and proteins. This property enhances their potential as delivery vehicles for targeted treatments.
Recent studies have unveiled the transformative potential of mesenchymal stem cells (MSCs) derived exosomes as ground-breaking drug delivery vehicles in the relentless battle against cancer and neurodegenerative diseases. Leveraging the intricate web of intracellular communication, MSC exosomes adeptly navigate the body, ensuring precise delivery of therapeutic cargo to targeted sites. These remarkable capabilities of exosomes show their potential to develop a highly effective treatment for incurable medical conditions, offering new hope to patients and researchers.
Current Technologies for Drug Loading
Effective drug loading in exosomes is crucial for enhancing therapeutic efficacy. Moreover, there are two types of loading mechanisms that are widely used for loading biomolecules into exosomes, as discussed below –
- Pre-loading – Before Exosome isolation
- Co-incubation – involves incubation of the therapeutic agent with parent cells
- Plasmid Transfection – involves transfecting exosome-generating cells with plasmids (having a sequence of interest) and isolating modified exosomes from the cells
2. Post-loading – After Exosome Isolation
- Sonication – utilizes ultrasound waves producing transitory holes to load cargo into exosomes
- Electroporation – provides external electric pulse ranging from 0.1 to 1000KV to create holes in exosomes; widely used for RNA encapsulation
- Freeze-Thaw – process includes freezing at -70°C followed by quick thawing to room temperature for numerous cycles for loading cargos
- Extrusion – exosome and cargo are pushed through 100-400nm pore polycarbonate membranes using an extruder with a heating block
- Saponin – enhances membrane permeability and loads small drug molecules in exosomes
Novel technology for cargo loading – EXPLORs technology – This novel technologyutilizes optic-activated reversible protein-protein interaction, wherein under blue light (460nm), CRY2-conjugated protein cargo briefly interacts with exosomes, which then internalize biomolecules through endogenous biogenesis.
Current Technologies for Surface Engineering
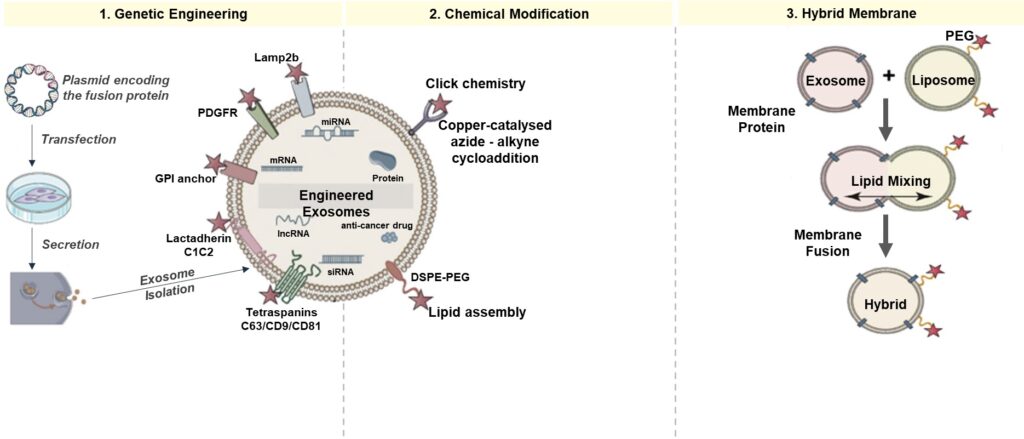
- Genetic Engineering
This surface modification empowers exosomes as precision-guided drug couriers, ensuring payload delivery exclusively to target cells and minimizing off-target effects. Examples include –
- Tetraspanins (CD63/CD9/CD81) – enhance stability and facilitate efficient cargo loading
- Lactadherin – enhances exosomes’ binding to phosphatidylserine on target cells
- GPI anchor – facilitates the attachment of specific proteins to exosome surfaces
- PDGFR – directs exosomes toward cells expressing the PDGF receptor
- Lamp-2b – enhances exosome endosomal escape and improves cargo release efficiency during cellular delivery
2. Chemical Modification
Chemical surface modification of exosomes tailors their surfaces, optimizing compatibility for therapeutic payloads and ensuring precise and efficient drug delivery to target cells.
- Click chemistry – Copper-catalyzed azide-alkyne cycloaddition directly attaches molecules to the surfaces of exosomes via covalent bonds
- DSPE-PEG – 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol), results in prolonged circulation and enhanced delivery
3. Hybrid Membrane
Exosome fused with synthetic liposomes containing PEG on their surface increase their circulation time in blood, decrease their immunogenicity, and reduces their uptake by mononuclear phagocytes cells.
Exosome Ligands and Receptors for Targeting/Labeling
Exosomes, although promising, sometimes lack precise targeting in clinical applications. Various targeting or labeling moieties have been utilized for precise strategies, such as,
- LAMP-2b/Tetraspanins are present in exosomal membranes that target peptides. For example, neurons, microglia, and oligodendrocytes in the brain can be targeted by fusing Lamp2b to the neuron-specific rabies virus glycoprotein (RVG) peptide.
- CP05 peptide,when conjugated with PEG2000 and coated on exosomes, has a high affinity for exosomal CD63 (tetraspanins), limiting exosome intake by the Mononuclear Phagocyte System (MPS) and establishing efficient delivery platforms for photodynamic/chemical cancer treatment.
- Lactadherin is used for in vivo tracking of exosomes by labeling with gLuc–lactadherin (gLuc-LA), a fusion protein of Gaussia luciferase (a reporter protein) and lactadherin (an exosome-tropic protein).
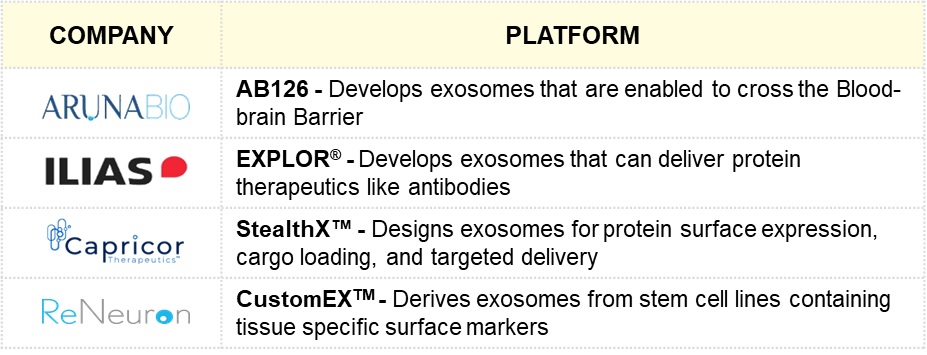
Recent Commercial Trends
Clinical Trials
- Nano24 – Their Phase II study includes anEXO-CD24 solution for Acute Respiratory Disease Syndrome (ARDS), with the solution’s efficacy and safety being tested on patients. [NCT05947747]
- Tehran University – Currently in Phase II, they developed human placenta exosomes made from mesenchymal stem cells that are also being tested for safety and effectiveness in treating complicated Anal Fistulae. [NCT05402748]
- Eskisehir Osmangazi University – They are investigating the efficiency of autologous synovial fluid mesenchymal stem cell-derived exosome’s application in patients suffering from a degenerative meniscal injury, with the Phase II clinical trial. [NCT05261360]
Related Posts
No posts found.