Cell & Gene Therapy in Oncology
Cell and Gene Therapies (CGT) are revolutionizing cancer treatment. These therapies provide personalized and innovative ways to tackle different forms of the disease. These treatment modalities offer the hope of finding exact and effective interventions for various pediatric, hematologic, solid tumors, and rare cancers.
Cell and gene therapies are sometimes described as ground-breaking treatments for cancer. They have the potential to cure many types of diseases, diminishing the underlying cause of genetic and acquired diseases.

Figure 1: Challenges and Opportunities
CGT has shown effectiveness across different cancer types, pointing to a glimmer of hope for patients with tough diagnoses. By targeting specific genes and cells tied with cancer, these therapies open up avenues for more tailored and accurate approaches, ushering in new hope among patients facing difficult diagnoses.
However, the journey is not without hurdles. Challenges such as genome editing complexities, patient unavailability, tumor heterogeneity, limited access to patient populations, etc., need strategic solutions. On the other hand, these challenges present many opportunities. New treatments that involve gene editing techniques designed against resistance mechanisms in cell and gene therapies, as well as advances in gene editing technologies, are laying a foundation for further breakthroughs.
Technologies like CRISPR/Cas9 gene editing, RNA therapeutics, Gene silencing technologies, CAR-T cell therapy, and Epigenome editing are at the forefront of this revolution. The precision of CRISPR/Cas9 makes it possible to target specific segments of genes with utmost accuracy.
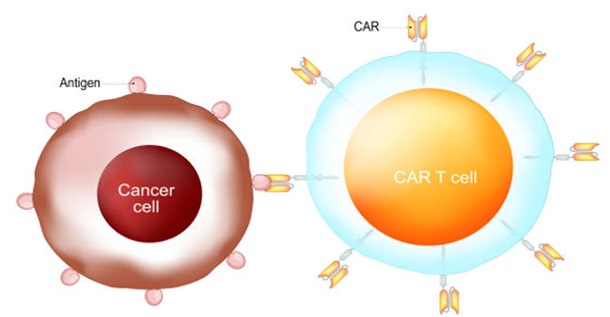
Figure 2: CAR-T Cell Therapy
CRISPR Therapeutics, Bluebirdbio, Intellia Therapeutics, Precision Biosciences, etc., are among the companies pushing the boundaries on gene editing for many diseases.
However, there is a gap in technology. For example, real-time monitoring of dynamic biomarkers, understanding optimal sequencing of cell and gene therapies, addressing resistance mechanisms, implementing automation and standardization, achieving precise delivery, etc.
Many emerging companies, such as XNK Therapeutics, NexImmnue, Capstan Therapeutics, Tessa Therapeutics, etc., have ventured into developing SGTs for cancer treatment in this dynamic environment. These ambitious approaches bring a level of flexibility and diversity in the search for efficient remedies.
Cell and gene therapy offers a transformative approach to cancer treatment, characterized by personalized and precise interventions. Cancerous genetics are addressed in these remedies, modified for specific situations, thereby reducing the risks of destroying healthy tissues or side effects. They give patients with difficult diagnoses a ray of hope for long-term remission or even a cure. Furthermore, they diversify the therapeutic inventory open to patients, especially those who suffer from recalcitrant or refractory cancers. Cell and gene therapy with advancing technology may enhance patient outcomes and permanently change the face of the cancer care landscape.
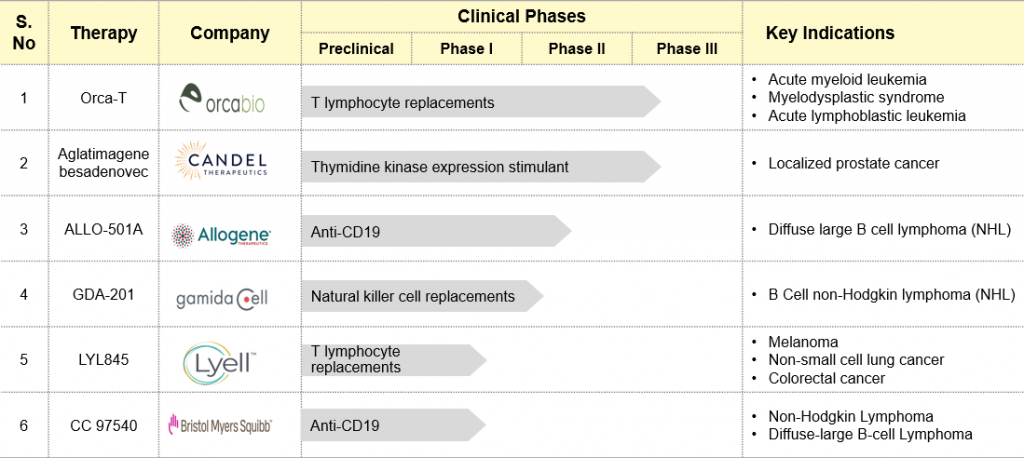
Table: Cell & Gene Therapies in the Clinical Stage
Therapies in development stages such as Orca-T, Aglatimagene besadenovec, ALLO-501A, GDA-201, LYL845, and CC 97540 highlight the continuous advancement in cell and gene therapy research. Moreover, recent FDA approvals underline the real-life impact of these developments. Among them is Adstiladrin (Nadofaragene Firadenovec-vncg), approved in 2022 for high-risk non-muscle-invasive bladder cancer. It is a non-replicating adenovirus vector-based gene therapy that introduces the interferon alfa-2b gene into the bladder to treat it differently.
Likewise, Breyanzi (Iisocabtagene Maraleucel), Abecama (Idecabtagene Vicleucel), and Carvykti (Ciltacabtagene Autoleucel) were endorsed between 2021 and 2022 as notable milestones as far as large B-cell lymphoma and multiple myeloma are concerned. These CAR-T cell therapies show how genetic alteration can be used to target and destroy tumor cells.
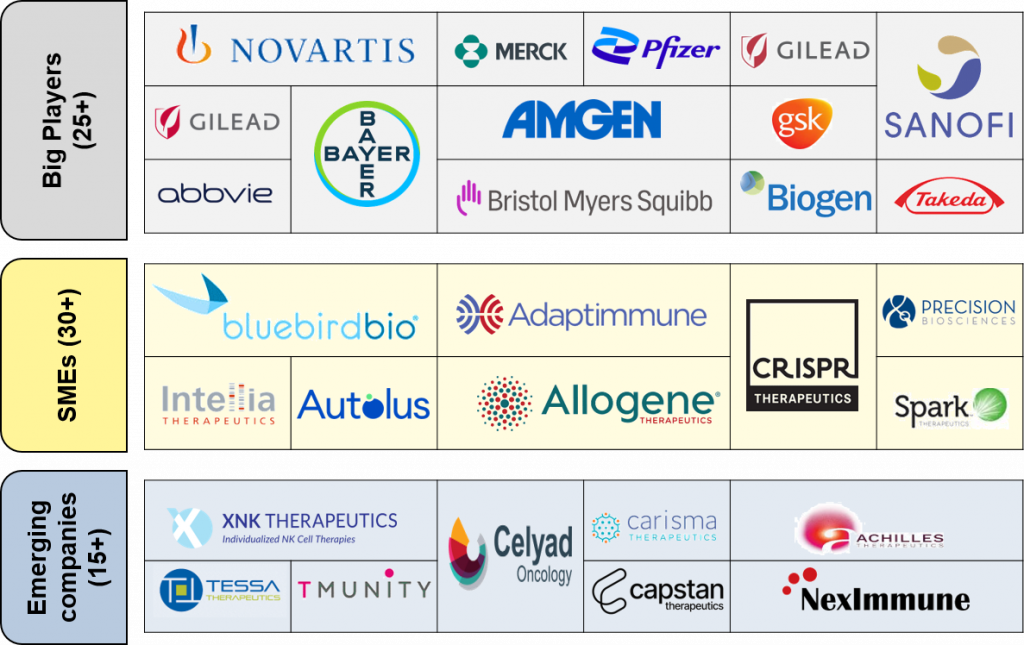
Figure 3: Market Players in Cell and Gene Therapies
There is a significant growth potential awaiting the global cancer gene therapy market as there is an increasing demand for this therapy, progress in the gene therapy space, and rising cancer rates worldwide. Nevertheless, challenges such as expensive treatments and robust regulatory approvals should be tackled to fully harness the CGT potential.
The market is characterized by the active involvement of key companies, including but not limited to Novartis, Merck, Pfizer, Sanofi, Bayer AG, Biogen, and AbbVie.
Collaborative endeavours and strategic investments, such as AstraZeneca’s collaboration with Cellectis, are being made to accelerate cell therapy and genomic medicine ambitions. Such collaborations are expected to fast-track the development and application of CGT in oncology.
Cell and gene therapy in oncology has a bright future as it comes with promising personalized treatment methods for cancer that can even cure it. Advances in gene therapy, including genetic correction, are opening new avenues for innovative treatments. However, regulating rare mutation cases and getting around with regulatory challenges are essential. Regulatory adaptation and collaborative efforts are needed to maximize these therapies’ potential and speed up their development for better cancer care.
The transformative effect of gene and cell therapy is altering not only how cancer is treated but also the idea of hope and healing. These pioneering methods give patients worldwide better accuracy and customization and a glimpse of being cured for once and all as they grow further.