Pharma Innovative Licensing and Access Pathway (ILAP)
In the world of pharmaceuticals, innovation is key. To keep pace with the rapidly evolving healthcare landscape, pharmaceutical companies are constantly searching for new ways to bring life-saving drugs to market faster and more efficiently. One promising approach that has emerged in recent years is the use of Innovative Licensing and Access Pathways (ILAP).
ILAP represents a paradigm shift in the way new drugs are brought to market, especially in the UK. Rather than relying solely on traditional regulatory pathways, ILAP leverages cutting-edge technologies and streamlined processes to accelerate drug development and improve patient and market access. From expedited reviews to expanded access programs, ILAP offers a range of tools and strategies that can help pharmaceutical companies bring new treatments to market faster and more efficiently than ever before.
In this article, we will explore the world of ILAP and its potential to transform the pharmaceutical industry. We will examine the benefits, processes, and features of the pathways to bring new drugs to market and offer insights into the future of this exciting new approach to drug development.
Pharma Innovative Licensing Background
The UK government introduced the ILAP in March 2021. This new pathway is designed to provide patients in the UK with faster access to innovative treatments and therapies by streamlining the regulatory approval process.
Under the ILAP, medicines that show promising and effective early-stage results in clinical trials can be approved for use in the UK more quickly than traditional licensing. This means patients can access new treatments sooner, even if the medicine has not been thoroughly tested and approved.
The ILAP is intended to encourage innovation in the healthcare sector and ensure that UK patients can access the latest and most effective treatments. Moreover, the government hopes this new pathway will make the UK a more attractive destination for investment in healthcare research and development, ultimately improving patient outcomes.
Agencies Collaborating in ILAP
- All Wales Therapeutics and Toxicology Centre (AWTTC):
- AWTTC is responsible for providing advice and support on the use of medicines in Wales and to ensure that patients have access to treatments and therapies in a safe and timely manner
- Medicines and Healthcare products Regulatory Agency (MHRA):
- MHRA assesses medicines’ safety, quality, and efficacy in the UK. MHRA reviews data from clinical trials and decides whether to grant a conditional marketing authorization
- National Institute for Health and Care Excellence (NICE):
- NICE is responsible for providing guidance advice on the use of medicines and treatments in the UK
- Scottish Medicines Consortium (SMC):
- The SMC is responsible for providing advice on the use of medicines in Scotland
- NHS England and NHS Improvement:
- These organizations are responsible for commissioning and providing healthcare services in England
- Health Research Authority (HRA):
- HRA is responsible for regulating and supporting health research in the UK. HRA works to ensure that the new pathway is implemented. It should be done in a way that supports high-quality research & protects the interests of patients.
- National Institute for Health Research (NIHR):
- The NIHR is responsible for funding and conducting health research in the UK
Overall, each partner in the ILAP plays a vital role in ensuring that patients in the UK have access to innovative treatments and therapies safely and timely while supporting high-quality research and protecting their interests.
ILAP Process and Eligibility
- Submission of initial application:
- Medicine developers can apply for the ILAP program at any product development stage. Initial application should provide a summary of the potential benefits and risks, & any existing clinical data
- Review by ILAP oversight board:
- The ILAP oversight board will review the initial application and determine whether the medicine is eligible for the ILAP program
- Dialogue phase:
- If the medicine is deemed eligible, the medicine developer will engage in a dialogue phase with the ILAP partners. This phase may involve discussing the medicine’s potential benefits and risks, as well as the proposed development plan & any additional data that may be needed
- Designation:
- If the medicine meets the criteria for one of the ILAP pathways (PIM, EAMS, or CMA), it may be granted a designation. The type of designation granted will depend on the stage of development, the strength of the existing data, and the potential benefit to patients
- Ongoing dialogue:
- Medicine developers with ILAP designations will continue to engage in ongoing dialogue with the ILAP partners. This may include providing updates on the development plan, sharing new data, and discussing any issues or concerns that may arise
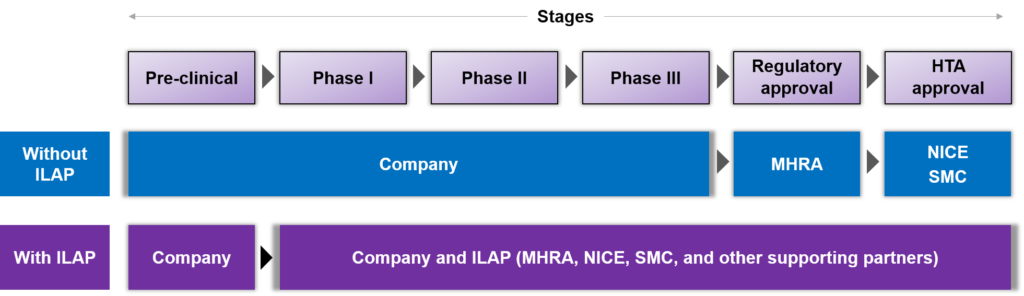
Figure 1: UK market access path with and without ILAP
Eligibility:
To be eligible for the ILAP program, a medicine must meet the following criteria:
- Address an unmet medical need; the medicine must be intended to treat a disease or condition for which there is no existing satisfactory treatment or for which the new medicine offers a significant improvement over existing treatments.
- Show promise of benefit; the medicine must have the potential to significantly benefit patients, such as improving survival or quality of life.
- To be in the early stages of development, the medicine must be in the early stages of development, such as phase I or phase II clinical trials. However, medicines in later stages of development may still be eligible if they meet the other criteria and are likely to benefit patients significantly.
- To Be scientifically valid, the medicine must be based on sound scientific principles and have a credible mechanism of action.
Once a medicine is deemed eligible for the ILAP program, it may be granted one of the following designations:
Promising Innovative Medicine (PIM) Designation:
This designation is granted to medicines that show promise for addressing unmet medical needs. PIM designation provides medicine developers with regulatory and development incentives to help speed up the development process.
Early Access to Medicines Scheme (EAMS):
This designation provides patients with life-threatening or seriously debilitating conditions access to promising new medicines before they are granted full marketing authorization, thereby leading to early access to new treatments.
Conditional Marketing Authorization (CMA):
This designation allows a medicine to be marketed and used in the EU before completing all necessary clinical trials. Also, available data must indicate that the benefits of immediate availability outweigh the risks of less comprehensive clinical data than normally required.
ILA Process Pathway
By utilizing the pathways based on non-clinical data, companies can enter the market very early, resulting in expedited access to innovative medicines for patients. This approach not only benefits companies in terms of quicker market access but also has the potential to accelerate the availability of potentially life-saving treatments for those in need.
Innovative Passport:
The Innovative Passport is a tool provided through the ILAP in the UK. It is designed to help medicine developers navigate the regulatory process and facilitate discussions with regulators. The tool summarizes the medicine’s development and data and provides a regulatory submission and review roadmap.
The eligibility criteria for an innovation passport include:
- Details of the condition, patient, or public health area
- The medicinal product fulfills one or more specific areas (based on what is mentioned in the application)
- The medicinal product has the potential to offer benefits to patients
The Innovative Passport consists of the following main sections:
- Overview: It provides a summary of the medicine’s development objectives, the target patient population, and the unmet medical need that the medicine is intended to address.
- Development plan: It outlines the medicine’s development plan, including the proposed clinical trials, the endpoints of each trial, and the data that will be collected. The development plan also includes the proposed timeline for regulatory submission and approval.
- Summary of data: It provides a summary of the existing medical data, including preclinical and clinical data. The summary should highlight any promising results or safety concerns.
- Risk management: It outlines the proposed risk management plan for the medicine, including any potential safety concerns and how they will be monitored.
Target Development Profile (TDP):
The Target Development Profile (TDP) is another tool that helps medicine developers and regulators align on the clinical development plan for medicine. The TDP also outlines the medicine’s development objectives, study endpoints, and data requirements. It is intended to help streamline the development and regulatory review process by ensuring that all stakeholders are aligned on the development plan.
It consists of the following main sections:
1. Background: This section provides a brief overview of the medicine’s development objectives, the target patient population, and the unmet medical need that the medicine is intended to address.
2. Clinical development plan: Outlines the proposed clinical trials for the medicine, including the study design, endpoints, and sample size. The development plan should be consistent with the medicine’s target product profile (TPP).
3. Data requirements: Outlines the data that will be collected in each clinical trial and the statistical analysis plan.
4. Risk management: Outlines the proposed risk management plan for the medicine, including any potential safety concerns and how they will be monitored.
5. Glossary: Provides definitions for key terms used throughout the TDP.
The TDP Toolkit:
Adaptive inspections:
These inspections support the non-clinical, clinical, and manufacturing aspects of a medicine’s development by conducting reviews at different stages of development through to post-authorization. Additionally, these inspections aim to transform regulation and ensure that patients receive medicines based on valid and reliable data.
Certifications:
This tool includes a rigorous review of different parts of the modules that make up the Common Technical Document (CTD). A multidisciplinary team at the Medicines and Healthcare Products Regulatory Agency (MHRA) will certify the modules and provide expert advice on the data and information required in the CTD. They will also highlight the strengths and weaknesses in different aspects of product development
Continuous benefit-risk assessment:
This tool provides ongoing advice to ensure that the agreed-upon development approach supports the collection of the most robust data to fulfill regulatory requirements and potential post-authorization commitments.
Clinical Practice Research Datalink (CPRD) Assisted Patient Recruitment:
This tool helps locate and potentially recruit eligible patients into clinical studies via a centralized search of the CPRD electronic health record database.
Enhanced Patient Engagement:
This tool facilitates the inclusion of patient perspectives in product development. Patient perspective is beneficial when it comes to discussing market access and may play a role in reimbursement.
The TDP toolkit supports evidence generation and ensures regulatory compliance for medicinal products. Each tool can be individually selected based on the product’s needs.
The ILAP also supports several pathways that can speed up the approval process for innovative medicines:
- Accelerated assessment: Designed to expedite the evaluation of medicinal products for severe conditions where there are unmet medical needs.
- Rolling review: Allows developers to submit data for regulatory review on a rolling basis rather than waiting until all data is available.
- Conditional marketing authorization: Allows the conditional approval of a medicinal product based on preliminary clinical data.
- Exceptional circumstance approvals: Allows temporary use of a medicinal product in exceptional circumstances, such as during an outbreak or pandemic.
- Access consortium: A collaboration between the European Medicines Agency and several European countries, providing early access to promising medicinal products for patients with unmet medical needs.
- Project Orbis: A collaboration between regulatory agencies in several countries that allows for simultaneous review and approval of a medicinal product across multiple jurisdictions.
Benefits of ILAP
ILAP offers several benefits to the pharmaceutical industry, including:
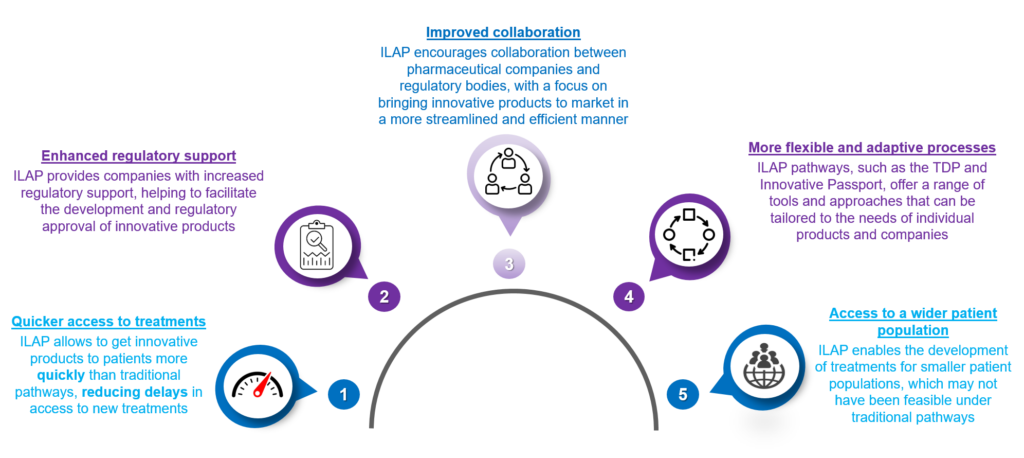
Figure 2: Benefits of Pharma Innovative Licensing
Overall, ILAP can help to accelerate the development and approval of innovative treatments, improving patients’ outcomes and benefitting the pharmaceutical industry.
When to Apply for ILAP?
It’s generally recommended to apply for the ILAP pathway as early as possible in the medicinal product development process to benefit fully from the program. The ILAP pathway accepts applications as early as phase 1, provided that non-clinical data is available. If companies wait until later stages of development, they may be unable to take full advantage of ILAP. Therefore, it’s advisable to consider the ILAP pathway early on in the development process if companies think their products are eligible.
How Much Does it Cost?
The fees for applying to the ILAP pathway in the UK are as follows:
- Innovation Passport fee: £3,624
- Development Profile (TDP) fee: £4,451
However, the fees of the TDP toolkit may vary depending on the specific medicinal product and the tools needed. These fees will be generally explained during the TDP process, which is part of the ILAP pathway.
ILAP Grants by Indications and Drug Phases
The ILAP grants are categorized based on the indication and regulatory agency designation. However, in 2021-22, Oncology was the most frequent target indication, followed by Psychiatry, Dermatology, Musculoskeletal diseases, Neurophysiology and Cardiology. A few assets that got ILAP grants are Belzutifan, Lorlatinib, Mobocertinib, Tepelizumab, Obecabtagene autoleucel, etc.
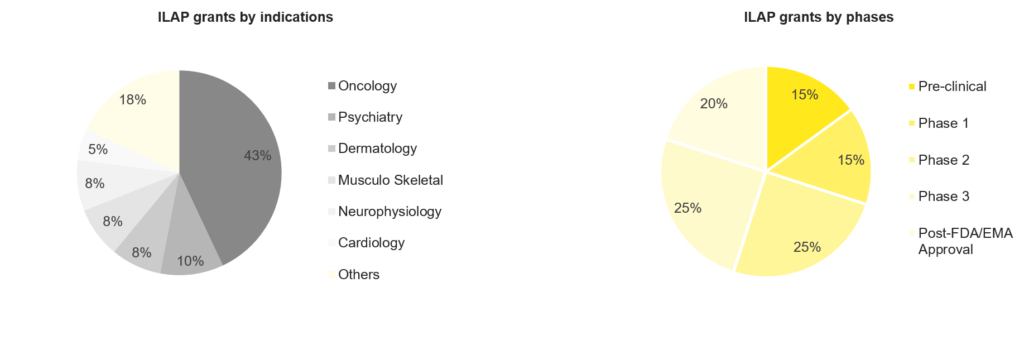
Figure 3: Statistics on ILAP grants
Based on the clinical development phase, the majority of the products were in the mid-stages of clinical development at the time of application or grant. Additionally, FDA and EMA-approved medicines accounted for approximately 20% of awardees. Pre-clinical development and phase 1 also represented a considerable amount of awardees.
Future of ILAPs
The future of ILAP is promising, as it aims to provide a more efficient and streamlined process for developing and approving innovative medicinal products. The ongoing development of new tools and pathways within ILAP is expected to enhance further the regulatory framework for innovative medicines in the UK and potentially serve as a model for other countries. Moreover, the continued collaboration between the government, regulatory bodies, industry, and patient groups will be crucial for the success of ILAP. Its ability to bring cutting-edge treatments to patients faster and more efficiently will be fruitful.
Conclusion
ILAP is an initiative designed to expedite the process of bringing new chemical entities, biological medicines, new indications, and repurposed medicines to market while facilitating patient access. The pathway utilizes horizon scanning and regulatory science to stay current with the latest developments and frameworks. Thus ensuring evidence-based practice in the face of new technologies and methods. The ILAP is available to both commercial and non-commercial medicine developers, both locally and globally, and consists of an Innovation Passport designation, a Target Development Profile (TDP), and access to a toolkit that supports all stages of the design, development, and approvals process.
The Innovation Passport serves as the access point to the ILAP. Also, it is open to developers from the pre-clinical trial stage to the mid-development program point, with a broad and inclusive definition of innovation encompassing new and repurposed medicines. Moreover, the passport is connected to a portfolio of activities by creating a product-specific Target Development Profile, with the evidence required for a product to meet the criteria depending on where it falls in the development pathway.