The quest for sustainable energy solutions has driven lithium-ion batteries (LIBs) to a significant level of technological advancement. The need for fossil fuels and non-renewable resources is rising mainly because of the simple fact they are needed to power everything from electric vehicles (EVs) to cell phones. In an attempt to compensate for this, enormous global manufacturing facilities are being built every day. There must be roughly 10 million electric vehicles on the road globally, which implies that resource depletion will accelerate and might result in environmental issues.
Consequently, a worldwide sustainable shift in the production of lithium batteries is necessary, as it is linked to a decrease in the use of non-sustainable resources through bio-based electrodes (anode/ cathode) solutions.
Therefore, a few advantages of bio-based alternatives are listed below:
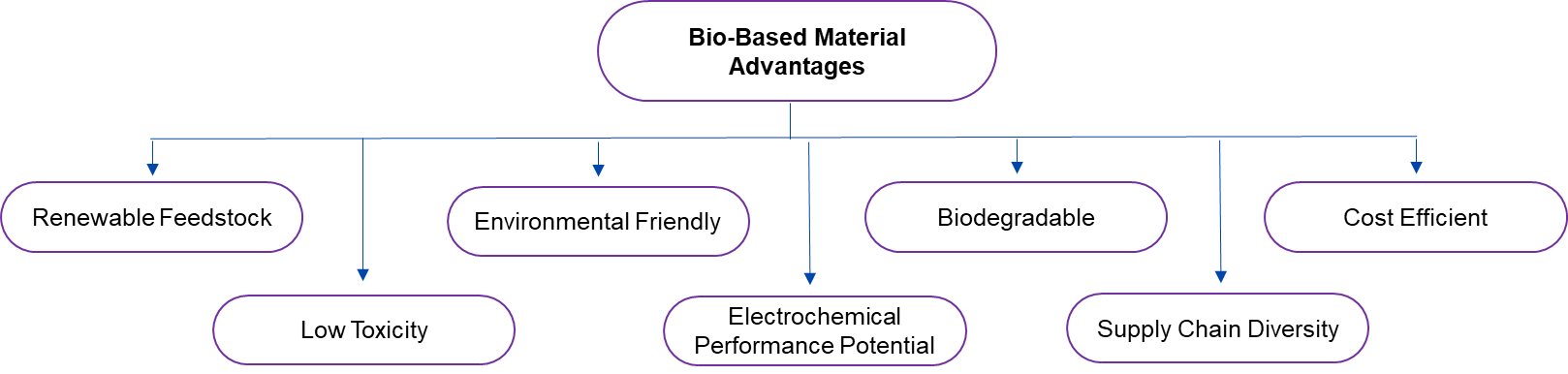
Figure 1. Technical Advantages Related to Bio-based Alternatives
Bio-Based Alternatives and Advancements for Anode
Anode materials used for lithium batteries are mostly titanium-derived compounds, silicon or silicon-derived compounds, carbon-derived materials (graphite), and carbon-silicon composite materials. Although all materials perform well electrochemically, they also have certain disadvantages. For example, silicon experiences volume expansion, and graphite has a poor energy density. Moreover, dendrites that grow from lithium metal anodes piercing the separator may result in a thermal runaway or explosion, endangering the surrounding environment and perhaps leading to battery failure.
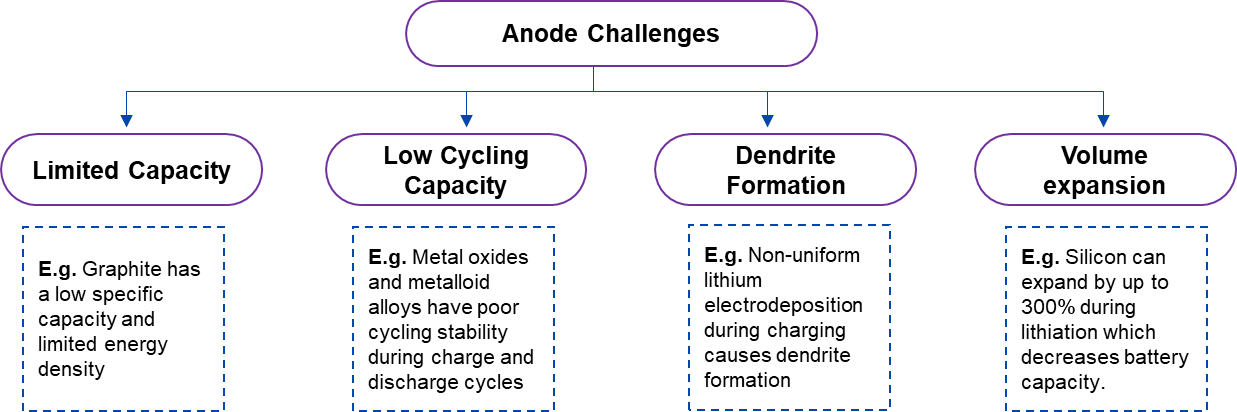
Figure 2. Technical Challenges Related to Conventional Anode Materials of LIBs
I. Biochar-Based Carbon Anodes:
Long-term energy storage can be achieved by using biochar-made lithium-ion battery anodes. The environmentally friendly biochar has a porous structure and large surface area, which facilitate lithium ion diffusion and provide plenty of lithium storage sites. Additionally, biochar is resistant to degradation during lithiation and delithiation, which ultimately improves the long-term performance and cycle stability of lithium-ion batteries. In addition, the following table highlights the different kinds of biochar materials (obtained from biomass feedstock) and their potential for use as anode materials in lithium batteries.
| Feedstock | Initial Battery Capacity (mA h g−1) |
|---|---|
| Mustard seed | ~822 |
| Rice straws | 2041 |
| Bagasse | 2347.56 |
| Banana peel | ~2150 |
| Wood | ~315 |
II. Biomass-Based Silicon Sources for Anodes
The elevated theoretical specific capacity is one of the many benefits of silicon anodes, yet there are several disadvantages, such as silicon expanding up to 300% during the lithiation process. For this reason, there is a need for bio-based silicon sources, such as agricultural wastes that are rich in phytoliths (comprising amorphous silicon dioxide). Silicon-based anodes, such as nanostructured silicon anodes, can be made using several methods. One method can involve the calcination of plants (at 550 and 800°C), which is followed by either acid or alkali leaching, and another method can be independent leaching. A wide range of natural silicon sources, such as rice husk, bamboo leaves, barley husk ash, bamboo charcoal, corn leaves, reed plants, horsetail plants, and sugarcane bagasse, have been explored for use as anodes in lithium-ion batteries.
III. Biomass–Based Carbon–Silicon Composites
Carbon-silicon composites are manufactured by mixing biomass-derived carbon with silicon in a uniform ratio utilizing several methods, including sol-gel or chemical vapor deposition. However, manufacturing carbon-silicon composites from renewable resources is tricky since surface functionalization is required to promote self-assembly. Therefore, various measures are necessary to create effective carbon-silicon composite-based anodes, including the requirement of high-quality biomass precursors, uniform distribution of carbon and silicon in composites, and formation of artificial solid electrolyte interface (SEI) layers on silicon before cycling to avoid the adverse reactions with the electrolyte.
| Biomass Source & Si Source | Anode Composition | Initial Discharge capacity/ current density (mA h g−1) | Retention Capacity |
|---|---|---|---|
| Rice husk and self-conversion to Si/C | Active materials + PVDF + Super Phosphorus (80:10:10) | 372.5 | 100%, 80 cycles |
| Rice husk (RH) and self-conversion to Si/C | C-Si RH + Super Phosphorus + Polyacrylic acid (60:20:20) | 1,554 | 100%, 200 cycles |
| Rice husk and calcination for self-conversion to Si/C | Si/C + Acetylene black + Sodium alginate (70:15:15) | 1368 to 398 (100 to 3000 mA g−1) | 500 mA g−1, 300 cycles |
IV. Tannin-Based Lithium-Ion Anode:
The hard carbon derived from tannin biomass may be a good alternative as an anode material since it enhances the material’s ability to store lithium. Hard carbon (HC) for lithium-ion anodes is produced by calcining tannin. HC had a columbic efficiency greater than 83.68% when used as the anode material in lithium-ion batteries (LIBs), and it exhibited a current density of around 400 mA g−1 after 200 cycles along with a capacity of around 218.1 mAh g−1. Although its application in lithium-ion batteries has not been thoroughly studied, tannin provides an ample supply for manufacturing hard carbon.
Bio-Based Alternatives and Advancements for Cathode Materials
The conventional lithium-ion battery cathode materials include Lithium Cobalt Oxide (LiCoO2), Lithium Nickel, Manganese Cobalt Oxide (NCM), and Lithium Iron Phosphate (LiFePO4). While each of these materials has specific advantages but, they also have certain disadvantages. For example, LiCoO2 and NCM have relatively small theoretical specific capacities and energy densities, which makes it difficult to withstand the high currents and quick charging rates required for grid storage and electric vehicle applications. Additionally, manufacturing or expanding the business requires a significant amount of resources, but this is further complicated by the price and availability of raw materials like nickel and cobalt, which limits their broad usage in large-scale applications. To meet the growing need for outstanding durability lithium-ion batteries for energy storage applications, development is underway to develop bio-based cathode replacements.
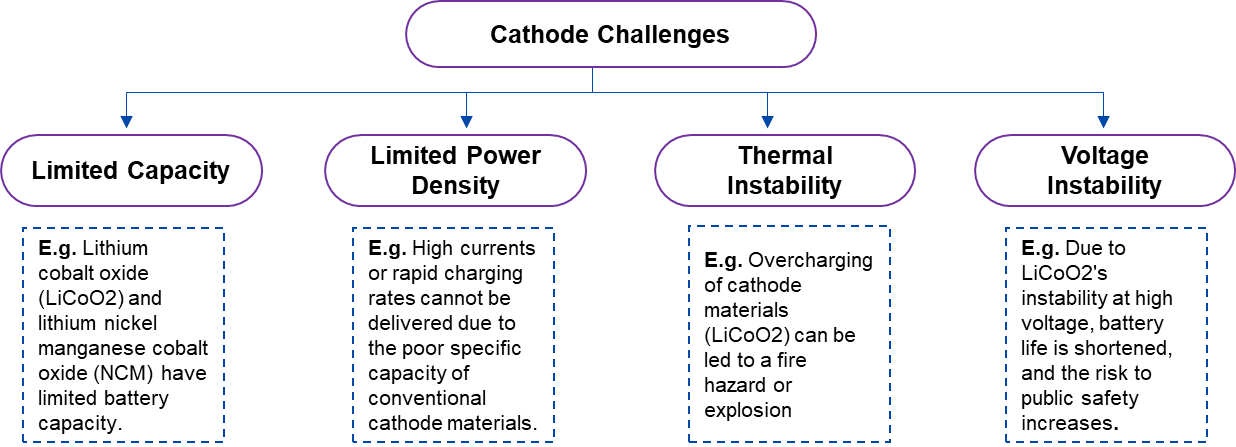
Figure 3: Technical Challenges Related to Conventional Anode Materials of LIBs
I. Bio-Based Sources of Iron And Phosphate For Sustainable LFP Cathodes:
Iron and phosphate must be sourced from bio-based or renewable sources to guarantee the sustainability of lithium iron phosphate (LFP) cathodes. The following are possible bio-based sources for each:
| Iron | Phosphate |
|---|---|
| Agricultural waste: rice husks, corn stover, wheat straw, coffee husks, or sugarcane bagasse | Agricultural waste: poultry manure, bone meal (dried animal bone powder), or crop residues |
| Biological organisms: Geobacter spp (Iron reducing and oxidizing bacteria) and Shewanella spp. (Iron reducing bacteria) | Biological organisms: Chlamydomonas pulvinata and Craticula molestiformis |
| Biomass: Chlorella vulgaris microalgae, seaweed or aquatic plants | Rock phosphate and recycled spent lithium iron phosphate batteries (around 40% P2O5) |
| Wastewater streams from iron or steel industries | Phosphorus-rich effluents like sewage sludge and animal production residual wastewater |
II. Lignin as both Cathode and Binder in Lithium Battery:
Lignin is a complicated organic polymer found in plant cell walls that is generated as a by-product of different sectors (such as paper or biofuel). Thus, lignin offers a compelling alternative to traditional carbon precursors, offering the possibility of increased energy efficiency, reduced costs, and lower environmental effects, i.e., lower CO2 emissions. The carbon generated from lignin is superior to conventional carbons based on polyacrylonitrile (PAN) in several ways. In addition, there are many different kinds of lignin, though we have only discussed a few of them and how they are utilized in lithium batteries.
| Type of lignin | Hydrolyzed lignin | Kraft lignin | Organosolv lignin | Acetone lignin |
|---|---|---|---|---|
| Role in battery | Cathode | Cathode and Binder | Anode | Anode |
| Discharge Capacity (mA h g−1) | 445 | Buckwheat-derived lignin: 600 Sunflower-derived lignin: 380 | – | – |
Furthermore, an overview of how lignin can be used as a binder or composite in LIBs is shown below:
| Lignin’s role in battery | Composition |
|---|---|
| Binder | LiNi0.5Mn1.5O4/lignin/graphite/acetylene black |
| Composites | Lignin/PEDOT hybrid composite cathode |
Additionally, electrochemical performance is shown below when lignin is used as a binder in lithium iron phosphate (LiFePO4) cathode for LIBs (where the anode is of graphite) in comparison to conventional binder such as Polyvinylidene fluoride (PVDF)
| Material | Reversible capacity (mA h g−1) | Retention capacity | Specific capacity (mA h g−1) | Columbic efficiency |
|---|---|---|---|---|
| Lignin bounded cathode | 148 | 94.1% after 1000 cycles | 110.8 | 99.5%. |
| PVDF bounded cathode | – | 46.2% | – | – |
Additionally, a hybrid composite comprising conductive Poly (3, 4-ethylenedioxythiophene) (PEDOT) and non-conductive lignin, present in an 8:2 composition ratio (PEDOT: Lignin), is tested in lithium-ion batteries, illustrating the potential to improve electrochemical performance as it displays a maximum capacity of around 50 mA h g−1, at a C/20 rate of cycling in electrolytes like LiPF6 and NaPF6 (higher than both lignin and PEDOT capabilities). Lignin can thereby increase the specific capacity and cycling stability of LiFePO4 cathodes.
III. Cobalt-Free Li-Ion Cathode Materials:
Lithium-ion batteries require cobalt, especially in the cathodes. However, the supply of cobalt is limited and hazardous, demanding the utilization of cobalt-free cathode materials. New technologies such as Lithium Nickel Manganese Oxide (LNMO) have the potential to produce energy densities of approximately 650 Wh/kg at the cell level and it is unique due to its ability to operate at high voltages along with cost benefits due to which lot of scientists are trying to maximize the LNMO’s electrochemical capabilities for lithium battery.
Current Market Developments in the Bio-Based Lithium-Ion Battery Electrode Field
Several organizations are promoting environmentally friendly advancements in lithium-ion batteries’ anode material and graphite production processes. Some companies, such as Birla Carbon, are introducing graphite sourced from bio-crude to achieve net zero carbon emissions by 2050. Furthermore, X-BATT also encourages carbon sequestration by forming a composite anode material out of agricultural waste items, such as bamboo, wood, maize husks, and rice husks, which are inexpensive carbon sources. Further, these composite materials exhibit higher reversible specific capacity and rate capability. Additionally, Stora Enso has introduced a new sustainable anode called Lingode® for lithium and sodium-ion batteries, made from lignin (a by-product of the pulp industry). This anode has a few advantages, such as faster charging/ discharging times and good performance in colder areas. Overall, these initiatives collectively offer a growth in the creation of environmentally friendly energy storage technologies for lithium-ion batteries. Regarding bio-based lithium battery anodes, research and market activity are mostly aligned toward replacing the currently used anodes with less scarce and cost-effective alternatives.
Conclusion
The development of bio-based anode or cathode material for lithium batteries for everyday use is still in its infancy. Still, it can bring about a change in sustainable energy storage systems. Additionally, an anode made from biomass-derived silica, carbon, or hard carbon can show good electrochemical performance and the potential to increase battery life with minimal environmental impacts. Furthermore, biomass-based cathodes with the potential to extend battery life are lignin-based cathodes. Thus, using bio-based anodes and cathodes will demonstrate a reduction in toxicity, carbon footprints, resource depletion, etc., supporting international efforts to reduce greenhouse gas emissions from electric vehicles or pollution resulting from chemically produced batteries. Furthermore, environmental experts must collaborate across disciplines to fully realize the promise of bio-based cathode and anode materials, as more research remains to be done to produce bio-based cathode/anode materials for use in daily life.