15 Minute City: The Sustainable Neighborhoods
15-Minute City was a concept devised by a French professor, Carlos Moreno, in 2016 to resolve mobility issues and improve convenience. According to his observation, in Paris, 66% of the public area is covered by cars, and their numbers account for more than 17% of the city’s population. The ideology behind the 15-minute city model was to provide cultural, economic, and social opportunities to residents within 15 minutes’ walking or biking distance.
If applied successfully, this kind of township can play a vital role in reducing carbon emissions by decreasing commute time and giving residents a better quality of life. It is a buzzing topic in every part of the world and a topic of discussion at COP27, which is about to conclude this weekend.
15-Minute City: Defining Features
Here are some salient features that define the importance and implications of 15-minute cities:
- Complete Neighborhoods: City planners can map the most essential amenities required by each community and work to provide easy access on a bike or foot. Importantly, this neighborhood design aims to alleviate everyday problems, not confine people to their immediate surroundings. Commuting to different parts of the city should be a choice and not a forced compulsion on a daily basis. Also, Portland, Oregon, has set a wonderful example in this context with a mapping analysis of a 20-minute neighborhood for the city.
- Green and Clean Space: The 15-minute townships will be very compact with green energy, buildings, circular resources, and an economy. Even the construction practices followed for these townships will be clean and eco-friendly. A classic example of this can be viewed in Milan, where L’Innesto is soon to become the first Zero-Carbon “Housing Sociale” district in its own country. It will feature a renewable energy-based heating system for neighborhoods and Nearly Zero-Energy Buildings.
- Promoting Health and Well-being: The 15-minute city concept not only revolutionizes urban planning but significantly contributes to individual health and well-being. By minimizing traffic, the 15-minute city reduces noise and air pollution, directly alleviating stressors linked to urban living. This transformative urban model enables residents to reclaim more personal time within their immediate communities, fostering a positive impact on mental health. Embracing walkable and bike-friendly neighborhoods aligns with healthier lifestyles, providing convenient options for physical exercise. In essence, the 15-minute city becomes a catalyst for both environmental sustainability and the holistic well-being of its inhabitants.
Additional Features for 15-Minute City
- People-Centered Mobility: An ideal compact city needs to benefit people of all ages and physical abilities. It will involve several measures, like enabling non-profit groups, businesses, and residents to turn around their on-and-off street parking spaces, improving pedestrianization of streets, etc. Moreover, active frontages on streets will create a positive atmosphere for bikers and walkers, supporting the local economy.
- A More Inclusive Environment: Apart from ecological and sustainability issues, the 15-minute city model resolves several basic problems related to urban life. It also brings equal benefits and opportunities for people of all races and economic groups. Modest-income families can relieve car ownership pressure as everything is within walking or biking distance. Some reports show that with this new model, an average New York family will be able to save USD $14,501 annually.
Implementation of 15 Minute City Model in Different Cities
Governments and city planners across the world are currently experimenting with this concept and trying to inculcate the idea of a green and safe lifestyle in people’s minds.
- Paris: Anne Hidalgo, Paris’s mayor, included the 15-minute city model in her 2014 re-election agenda. Considering the compactness of this dense, historic city, she suggested “hyperproximity” to connect all car-centric areas with cycleways. In addition, it was a smart move to facilitate trade and good health among citizens. After 8 years, Hidalgo continues leading the project, creating a safer, greener, healthier environment for Parisians.
- Melbourne: The City of Melbourne is using a 20-minute neighborhood model that includes the development of walkable communities for playing, working, and living. The city planners aim to invest in better public transport infrastructure and mixed-use public spaces. If successful, it will reduce Melbourne’s greenhouse emissions by 370,000 metric tons every day. They also plan to create cooler streetscapes and help the city reach its net zero emission plans by 2050.
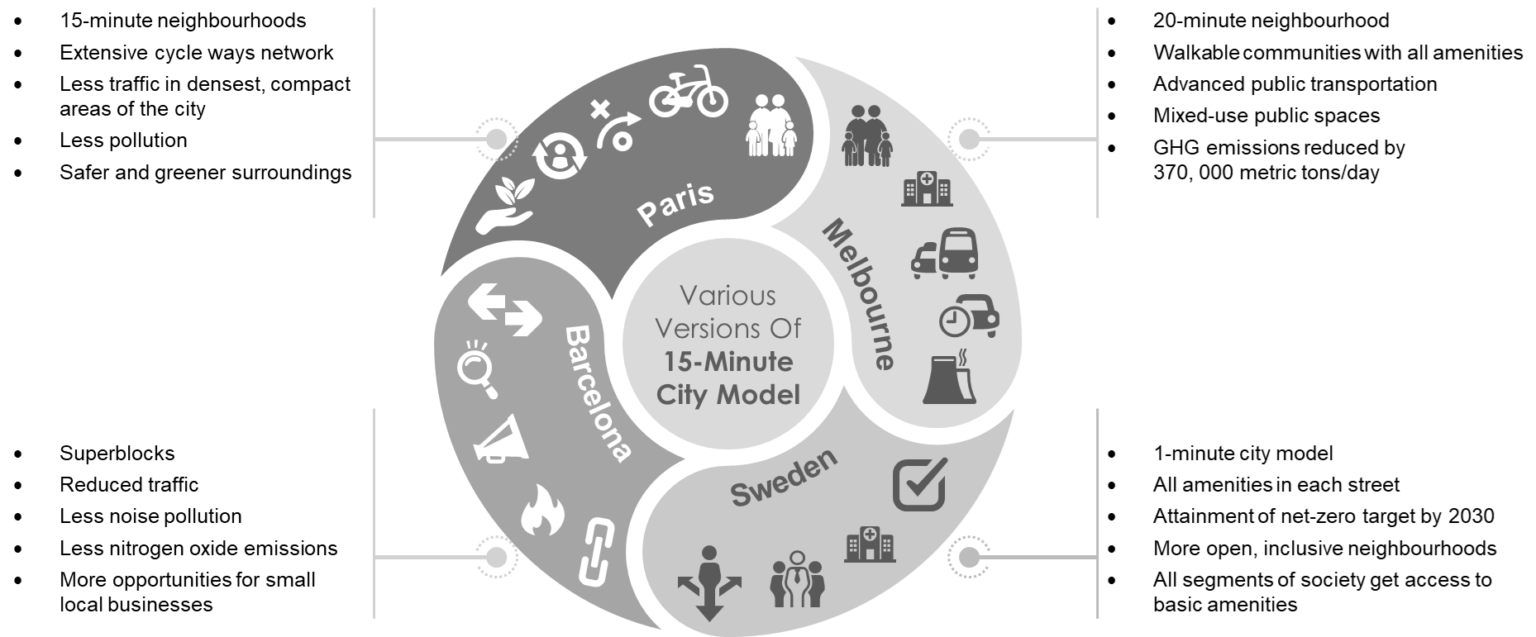
Figure: Various Versions of 15-Minute City Currently Being Implemented All Over The World
Other Important Cities
- Stockholm: Sweden took things a step ahead with the 15-minute model by upgrading the development of intersections and streets. Vinnova, the innovation agency, is focusing on providing all amenities on each street and connecting all residents with their built environment. In addition, they call it the “1-minute city model”, which, if successful, will help nine other Swedish cities besides Stockholm to reach their net-zero emissions target by 2030.
- Barcelona: Barcelona’s urban planners introduced “superblocks” in 2016 to experiment with the idea of a 15-minute city model. They created clusters of nine city blocks, forming one “superblock” that confined traffic within its perimeter. Motorists expressed strong opposition. However, implementing this plan effectively reduced nitrogen oxide and noise pollution within a short span. It also gave local small businesses a better chance to thrive near pedestrian areas. Moreover, with time, they proved how the redevelopment and pedestrian schemes were a win-win for all sides.
Conclusion
The 15-minute city model is one of the first of its kind, which can make future smart cities globally. It lets the idea of a green, peaceful, and economically viable life settle down in everyone’s imagination. Everyone will reap its benefits, especially those who need them most. Moreover, the idea focuses on those quintessential elements that define a quality life rather than things that just complicate and burden families with endless expenses. It refocuses attention on the living rather than non-living entities, initially completing the circle of life as nature intended.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.