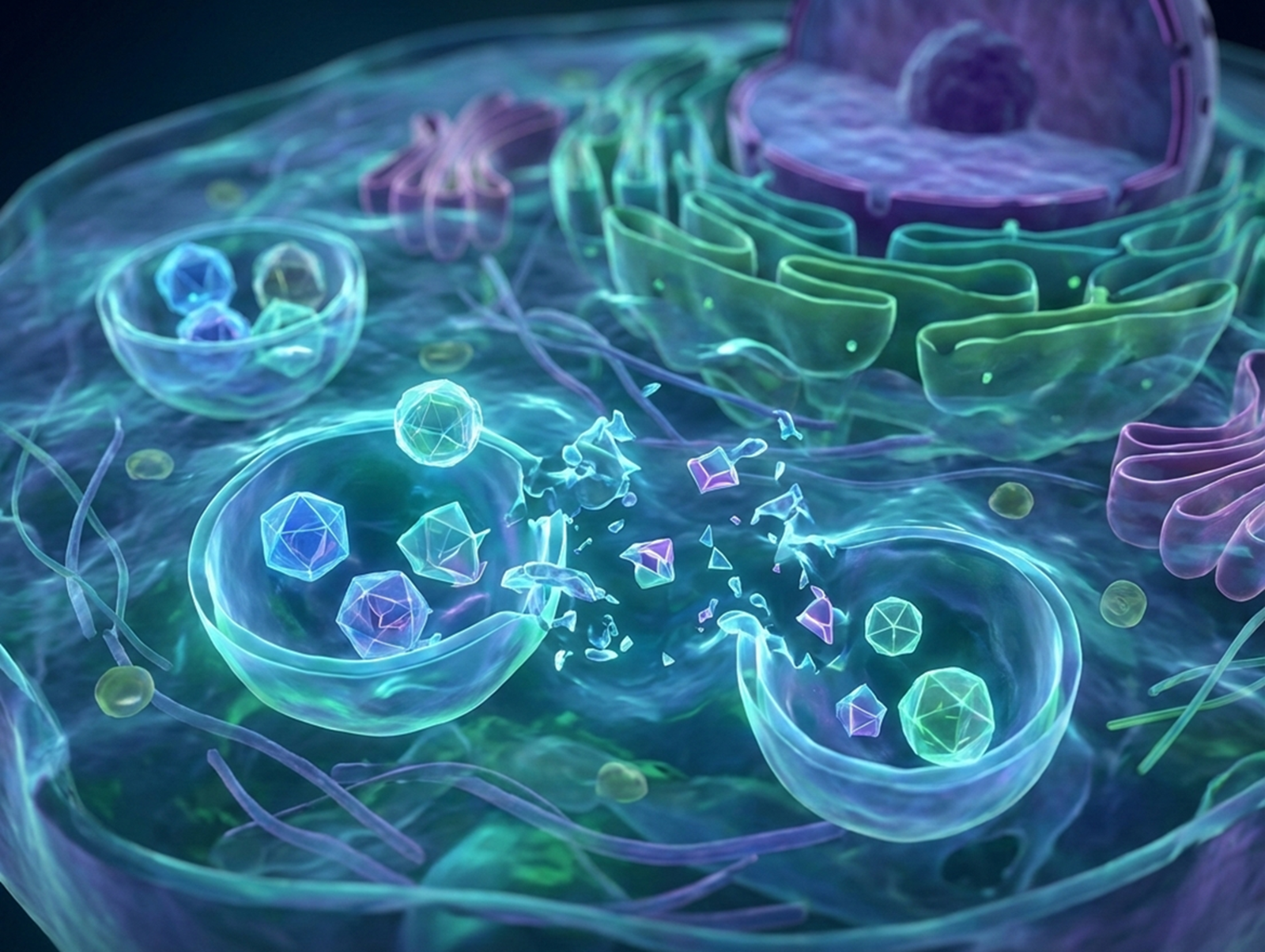
Advanced Therapeutics Hub: Strategic Intelligence for Next-Generation Biopharma
Executive Summary: The Frontier of Next-Generation Medicines
The worldwide healthcare and life science industry is changing its structure fundamentally. Traditional small-molecule drugs and standard biologics are being supplemented and, in some instances, replaced by advanced therapeutics that mend the damage at genetic, cellular, and molecular levels.
The transformation to the future of healthcare is largely due to the convergence of RNA therapeutics, mRNA platforms, cell and gene therapies, tissue engineering, precision medicine, targeted cancer vaccines, and novel drug modalities such as proteolysis and antibody drug conjugates (ADCs, targeting chimeras (PROTACs). These solutions are redefining the treatment paradigms by shifting the focus from providing symptom relief to correcting the root cause of the disease.
Together, these technologies open up the healthcare industry to unfathomable possibilities for rare, chronic, and previously untreatable diseases; thus, advanced therapeutics are the foundation for next-gen healthcare frameworks.
Introduction: Transformation of Biopharma
How Therapeutics are Evolving?
The pharmaceutical industry has transitioned from a chemistry-driven approach to a biologically driven innovation. The first biologics merely enhanced the specificity; however, the current advanced therapeutics have the ability to even reprogram biological systems to repair, replace, or regulate the malfunctioning pathways with high precision.
Emergence of a Multi-Modal Therapeutic Ecosystem
The industry is not only single, but modality solutions also dominate now. To put it simply, multiple therapeutic formats such as RNA, proteins, cells, vectors, and antibodies are being developed side by side; most of them are being designed to work together throughout disease stages.
The Convergence Paradigm: Toward a New Era of Medicine
The centre of gravity in biopharmaceuticals has been shifting towards a shared and programmable therapeutic stack, wherein the payloads (genes, RNAs, proteins), delivery systems, and targeting biologics are no longer siloed therapies, but interoperate. The uniqueness lies not in the progress of each modality but in the potential they showcase, synergistically. These innovations are driving the global biopharmaceutical industry towards a multi-modal therapeutic ecosystem, addressing diverse angles simultaneously.
The infrastructure, while enabling convergence of biopharma, highlights key aspects, such as –
- Advancements in Nanomedicine and Genetics have enabled RNA therapeutics with scalable manufacturing and swift iteration, along with rapid and on-demand expression or silencing of genes, payloads in shared delivery vehicles, and biomarker-guided precision strategies.
- Tissue engineering, along with the localized gene and RNA delivery within scaffolds, drives regeneration and functional integration of the tissues, while bridging structural repair and molecular therapeutics together.
- Targeted Cancer Vaccines and various platforms have been synchronized with checkpoint inhibitors and ADCs for coordinated immune and cytotoxic action.
The convergence is transforming medicine into a multi-modal ecosystem, having modular, adaptive, and synergistic therapies.
Core Modalities Landscape
The future of advanced therapeutics is becoming more and more about highly precise, platform-based modalities that can control disease at the molecular, cellular, and even systems level. The main players in the field are RNA therapeutics, cell and gene therapies, cancer vaccines, antibody engineering, targeted protein degradation technologies, and others. Interestingly, these different technologies are converging by using common delivery architectures, standardized manufacturing frameworks, and regulatory pathways that are changing.
Such a convergence opens the door for modular therapeutic design, scalability of production, and rational combination strategies, which in turn allow biopharma companies to fine-tune efficacy, safety, and durability, as well as speed up translation across oncology, immune, and genetically driven diseases.

1. RNA Therapeutics & mRNA Medicine
RNA therapeutics have become a fundamental part of the next wave of biopharma, as they allow for the programming, transient, and highly adaptable regulation of biological systems without the need for permanent genomic changes. The improvements in RNA chemistry, delivery methods, and manufacturing standardization have elevated mRNA from a vaccine platform of a small field to a broadly applicable therapeutic modality in oncology, genetics, immunology, and regenerative diseases.
- mRNA Therapeutics: Cytoplasmic non-integrating mRNA allows for rapid and easily controlled protein production; IVT manufacturing is scalable, LNP delivery is established, and there is regulatory familiarity, thus pharma companies can reuse the infrastructure across different indications, which positions mRNA as the core programmable therapeutic platform.
- Circular RNA (circRNA): RNA molecules that are covalently closed are resistant to nuclease and can support prolonged protein expression, thus the dosing frequency is reduced. Although circularization and QC are more complex, circRNA uses the same delivery systems as strategically suited for chronic and regenerative applications.
- mRNA-Based Gene Editing: Transient mRNA delivery of gene editing enzymes enables precise genetic modification with reduced long-term safety risk compared to DNA vectors. The use of shared mRNA CMC, delivery platforms, and regulatory pathways is facilitating the in vivo gene editing translation at a faster pace.
- Self-Amplifying RNA (saRNA): In saRNA, the replication machinery is one of the encoded parts, and intracellular RNA is thus amplified, obtaining very high protein output from a very low dose; this improves cost efficiency and global scalability despite the difficulties in delivery due to larger constructs, particularly for vaccines and immune therapies.
- Personalized mRNA: The possibility of treatment customization within a large-sized programmable RNA ecosystem is facilitated by rapid digital-to-manufacturing workflows, adaptive regulatory models, and precision immunotherapy. These factors are enabled by a patient’s mRNA and the specific nature coding for tumor neoantigens.
2. Cell & Gene Therapy
Cell & gene therapies are transitioning from mainly ex vivo, bespoke manufacturing to more in vivo, platform-based delivery models. This change is lowering the cost, simplifying the logistics, and enabling scalable deployment. Besides, the integration of gene editing is widening the safety, durability, and the number of non-oncology indications.
- In Vivo CAR-T: A CAR-T generation method, which involves programming the cells in vivo rather than processing them ex vivo, leads to a reduction in the time from vein to vein, the complexity of the manufacturing, and logistics. At the same time, it paves the path for standardized delivery and integration with gene editing tools for persistence and scalability.
- Oncology Applications: The most clinically mature and commercially viable area for cell therapies remains oncology. It is benefiting from standardized protocols, established regulatory precedents, and a deep competitive pipeline in both hematologic and solid tumors.
- GVHD & Safety Optimization: The use of gene editing and immune engineering to mitigate graft-versus-host disease and off-target immune activation has become a prominent theme in the research of safety concerns that have been a major obstacle to the wider dosing and repeat administration.
- Expansion beyond Oncology: The development of platforms and the improvement of safety profiles are allowing cell & gene therapies to move into autoimmune, inflammatory, and rare genetic diseases, where immune modulation that is durable and gene correction provides therapeutic value in the long term.
- Integration with Gene Editing Platforms: CRISPR and other gene editing technologies are becoming an integral part of cell therapy workflows, which allows multiplex editing, increases the specificity, and provides programmable control. This, in turn, is making cell and gene therapy more powerful and more compatible with the larger programmable therapeutic stack.
3. Cancer Vaccines
Cancer vaccines are gaining traction again as a significant immunotherapy class in the clinic, largely due to AI-driven neoantigen discovery, rapid manufacturing platforms, and the use of combination-based treatment strategies. Modern cancer vaccines are less of a standalone therapy and more of a programmable immune, priming tool that can be integrated into broader oncology treatment ecosystems.
- Manufacturing Model & Platform Shift: The cancer vaccines have changed from being protein-based complex ex vivo-like workflows to mRNA and viral vectors, which are in vivo-acting platforms. This has facilitated rapid, scalable, and digitally driven manufacturing with a lower logistic burden.
- Standardization & Logistics Consolidation: The platform-based vaccine production (especially mRNA) enables the reuse of delivery systems, analytical assays, and regulatory frameworks, thus supporting faster turnaround times and global deployment despite individualized components.
- Safety & Immune Control Considerations: Cancer vaccines, unlike cell therapies, do not have risks such as GVHD but need precise immune activation control to avoid systemic inflammation and off-target immune toxicity, especially in combination regimens.
- Competitive Maturity in Oncology: Melanoma, lung, and gastrointestinal cancers are the most advanced clinical landscapes and have strong competitive pipelines and increasing vaccines’ validation as combination partners rather than monotherapies.
- Expansion beyond Oncology: The platform maturation and immune targeting insights place cancer vaccine technologies as the next generation of therapeutics for chronic viral infections and immune-mediated diseases.
- Integration with Other Modalities: Cancer vaccines are progressively paired with PD, 1/PD, L1 inhibitors, ADCs, and cellular therapies, where they serve as immune primers that elevate response depth, durability, and patient stratification in multi-modal treatment regimens.
4. Antibody Engineering & ADCs
Antibody engineering has now become a high-growth industry segment due to the innovations in linker chemistry, cytotoxic payloads, and precision targeting. These innovations have made ADCs and bispecific antibodies clinically validated and commercially attractive platforms. Moreover, they are increasingly being placed as backbone therapies in combination treatment strategies.
- Antibody–Drug Conjugates (ADCs): Next-generation ADCs utilize both cleavable and stable linker technologies along with very potent payloads that improve tumor selectivity and therapeutic index; the rising number of regulatory approvals and the strong licensing activity are indications of ADCs’ maturity and their wider role in combination regimens beyond oncology.
- Bispecific ADCs: Bispecifics are also in development but have already caught the attention of investors due to the promising efficacy and safety they might have; they achieve the specificity of targeting by engaging two different antigens or by combining targeting with immune modulation—thus, there is a marked reduction in off-target toxicity.
- Engineered Antibodies: Next-gen antibody formats have significantly improved binding specificity, immune engagement, and half-life; enhanced safety and manufacturability have spurred clinical advancement and collaborations in oncology as well as immune-mediated diseases.
Emerging Modalities PROTACs
PROTACs, or Proteolysis-Targeting Chimeras, have transformed the approach to drug development by allowing the selective degradation of disease-related proteins instead of just temporary functional inhibition. They use the ubiquitin-proteasome system of the cell, which makes it possible to use cancer cell killing mechanisms that were thought to be non-druggable, like transcription factors and conduit proteins.
Despite the existence of E3 ligase diversity, tissue selectivity, and pharmacokinetics as some of the hurdles, tremendous progress is being made, and new clinical trials are being opened up in areas like cancer, brain disorders, and immune diseases. New methods, such as smaller molecules, new E3 ligases, and the use of RNA and antibodies for delivery, are making PROTACs part of the next-gen multi-modal and programmable therapeutic ecosystems.
Opportunity Landscape
As the advanced therapeutic hub captures the convergence of cutting-edge biopharmaceutical modalities into a shared ecosystem, this creates various opportunities across.
- Scientific innovations uncover opportunities in cross-modality synergies, wherein these modular and updatable biologics can be developed in tandem while reducing redundancy.
- Clinical transformation has been actively moving beyond symptom management to root-cause correction, involving personalized therapies, biomarker-guided strategies, real-time monitoring, and combination strategies.
- The healthcare system presents varied opportunities with preventive medicine, involving early intervention using genetic and molecular tools, global accessibility in limited-resource settings, and data-driven ecosystems integrating diagnostics, treatment, and monitoring.
- The strategic vision empowers the biopharma companies to build a shared infrastructure fostering collaborations, investment opportunities, scalable manufacturing facilities, and interdisciplinary hubs, accelerating growth.
This is not just a research cluster – but a living ecosystem for offering opportunities converging medicine, innovation, and building resilient healthcare outcomes. And while it offers immense opportunities, the path forward is marked by significant challenges.
To ensure that the breakthroughs remain safe, affordable, and accessible, the sustainable ecosystem must be formulated by combining scientific rigor, regulatory flexibility, economic innovation, and digital integration – remodelling the future of healthcare.
Challenges across the Industry
- Scientific and Technical Challenges: Specificity of target/specificity of targets (off-target gene editing/immunogenicity), payload stability, durability of response; all of these represent major challenge areas for RNA therapeutics, In Vivo Gene Therapy, and Multi-Modal Treatment Constructs (architecture).
- Clinical and Regulatory Hurdles: Most legacy designs/market-based/indication-specific trial designs are not optimally designed for Personalized/Adaptive/Combination therapies, and therefore create considerable complexity when assessing the benefits vs risks and implementing long-term follow-up.
- Manufacturing and Economic Barriers: Manufacturing processes that require GMP are complicated to operate on due to limitations in: Availability of Vectors, Availability of World Supply Chain (LNP), Cold Chain Requirements, and limited quantity of standardised platforms. All these limitations will cause issues with Scale, Cost Reduction, and Global Accessibility.
- Digital and Data-Integration Challenges: As Genomic, Clinical, and real-world Data sets are often maintained within individual silos, they are unable to effectively leverage AI-based Patient Stratification (using many of the same sources/types of Data), Data-based Predictive Modelling, Real-Time Pharmacovigilance, and Closed-Loop Therapeutic Optimization.
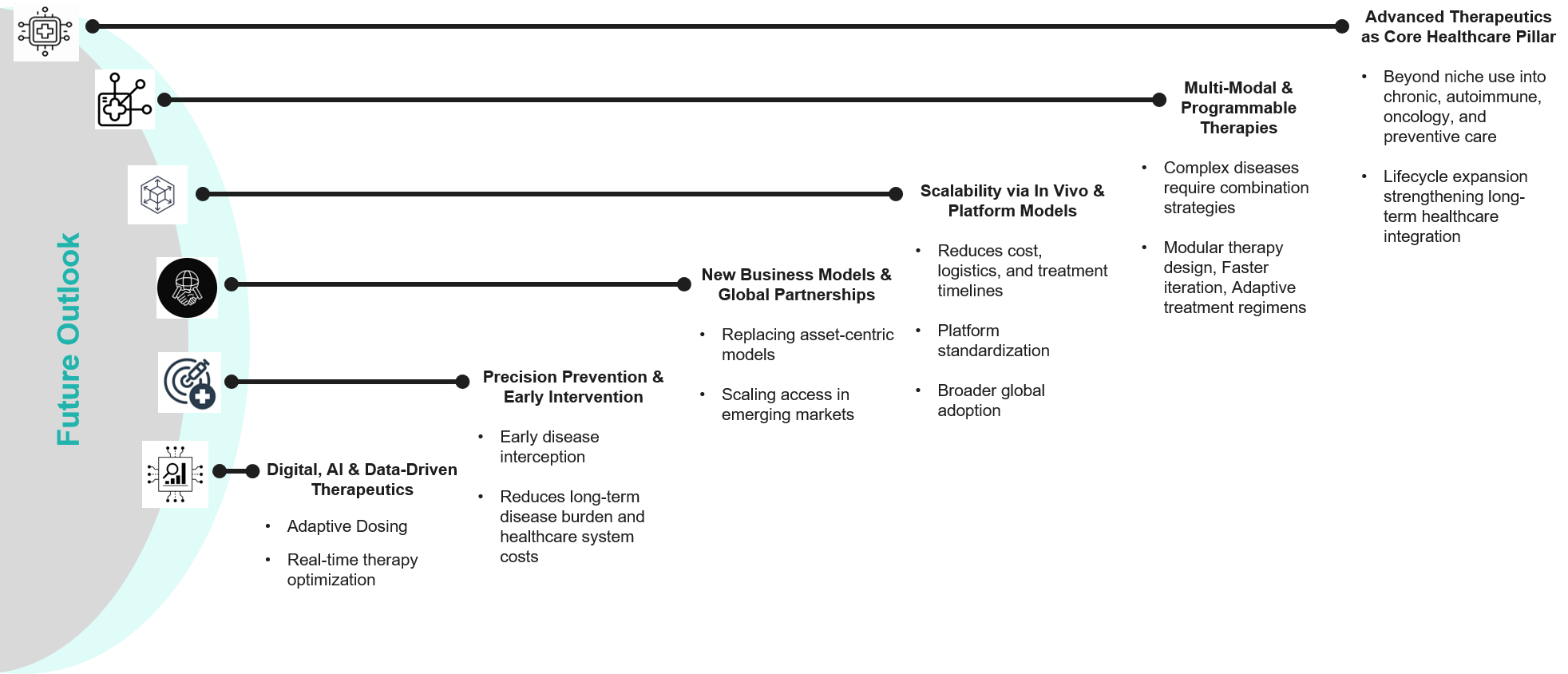
Future Global Perspectives
The perspectives highlight a future where these advanced therapeutics are not only niche innovations but the foundational pillars of global healthcare. Personalized and Accessible Healthcare systems will have the economies to thrive on biotech-driven growth and science that will be pushing boundaries with programmable and multi-modal therapies.
This shift into multi-modal orchestration of individual therapies and modalities will create new business models, foster global partnerships, and drive economic resilience – marking the dawn of a new era in global healthcare.
Stellarix Capabilities
Stellarix facilitates next-generation biopharma innovation by simplifying complex science into technology, clinical, and commercial strategies that can be easily implemented across advanced therapeutic platforms.
Stellarix features a range of technology, centered capabilities, including:
- IP, Competitive & Partnership Mapping – Analysis of patent landscapes, startups, pharma pipelines, and partnership opportunities to facilitate licensing and co-development decisions.
- Delivery & Targeting Strategy – Advising on nanoparticle systems, vectors, antibody targeting, and tissue-specific delivery to not only enhance efficacy but also ensure safety and biodistribution.
- Manufacturing & CMC Readiness – Recommendations on platform standardization, GMP scalability, cold chain optimization, and cost-efficient manufacturing pathways.
- Regulatory & Risk Intelligence – Identifying regulatory precedents, safety risks, long, term follow, up requirements, and approval pathways in various international markets.
- Investment & Commercial Strategy – Combining technical and market due diligence, value creation roadmaps, and market entry tactics to target emerging and established therapeutic platforms.
- Clinical & Translational Strategy – Assessment of preclinical models, biomarker strategies, dosing paradigms, and combination therapy positioning as a means of increasing translational success.
- Platform & Modality Intelligence – Detailed technical evaluation of RNA therapeutics, LNP delivery, gene editing, ADCs, PROTACs, and cell therapies to pinpoint scalable and differentiated approaches.
By adopting this comprehensive, technology-oriented strategy, Stellarix empowers life science and biotechnology executives to lessen the risks associated with innovation, speed up the development process, and create a sustainable competitive advantage across the advanced therapeutics ecosystem.
Conclusion
The biopharmaceutical industry is not staying with the traditional drug development process, which is single-modality and symptom-centric. It is now moving towards advanced therapeutic platforms that can treat diseases at the genetic, cellular, and molecular levels. Legacy approaches, which were mainly small molecules and first-generation biologics, were limited in terms of the duration of treatment, precision, and flexibility.
Conversely, the new landscape is characterized by programmable and multi-modal systems that incorporate several targeted protein degradation technologies, including RNA therapeutics, CGTs, cancer vaccines, and ADCs. Recent advancements in AI-driven discovery, delivery science, standardized manufacturing, and adaptive regulatory models have allowed these platforms to support scalable combination strategies and personalized intervention. The convergence of advanced therapeutics is not only taking place but also transforming experimental innovations to become the foundation of global healthcare delivery, economic models, and long-term disease management.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.