In-vivo CAR-T Cell Therapy: New Frontier in Cancer Treatment
Chimeric antigen receptor T-cell (CAR-T) therapy transformed oncology by paving the path for harnessing patients’ own cells to treat B-cell lymphomas, leukemias, and other hematological malignancies. Traditionally, CAR-T cell therapy for cancer relied on ex vivo processes, where a patient’s T-cells are extracted, genetically reprogrammed in an external medium to express synthetic receptors targeting cancer cells, expanded, and then reinfused into the patient’s body. It was a remarkable approach that yielded unprecedented results, but its scope was restrained by technical, financial, and logistical constraints that restricted its scalability and accessibility.
This paved the path for a more modified approach, in-vivo CAR-T cell therapy, where the immune cells of the patient are directly reprogrammed within the body, bypassing the need for complex cell manufacturing. This emerging approach promises to overcome the pertaining challenges and write a new chapter in T-cell therapy for cancer. This article explores the modus operandi, clinical applications, technological advancements, challenges, and future direction of in-vivo CAR-T therapy as the next milestone in cancer treatment.
How Does It Work?
In-vivo CAR-T therapy employs viral vectors or non-viral delivery systems that deliver genetic instructions for CAR expression directly to T cells within the host’s or patient’s body. The most commonly used methods include:
- mRNA-LNP Technology: Inspired by mRNA vaccines, LNPs are packaged with mRNA sequences that encode the CAR construct. These specifically designed LNPs target T cells, and once internalized, the mRNA is translated into CAR proteins, reprogramming the T cells to identify and attack cancer cells.
- Imaging & Monitoring: A few other approaches include the use of a second mRNA component that encodes traceable proteins, allowing for the real-time tracking of CAR-T cell generation and distribution using PET imaging.
- Dosing and Repetition: Unlike conventional CAR-T therapy, in vivo techniques enable repeated administrations, thereby enhancing efficacy and eliminating the need for additional cell manufacturing or extraction.
| Feature | Ex-vivo CAR-T Therapy | In-vivo CAR-T Therapy |
| Engineering Venue | Laboratory | Inside the patient’s body |
| Manufacturing | Weeks-long, very complex, patient specific | Simple with off-the-shelf potential |
| Total Cost | ~$300,000-$500,00 per treatment | Relatively lower |
| Lymphodepletion | Required, which increases the risk of infection | Not required |
| Toxicity Management | Dose adjustment is possible due to real-time monitoring | Inside the patient’s body |
| Current Application | Hematological malignancies | Expanding to solid tumors, autoimmune diseases, and so forth |
| Customization Scope | High | Low |
| Accessibility | Limited | Potentially broad |
| Primary Challenges | Manufacturing, time, cost, and logistics | Targeted delivery, efficacy, safety |
Clinical Applications of In-Vivo CAR-T Cell Therapy
Hematological Malignancies
- B-cell Lymphomas and Leukemias: In vivo CAR-T cell therapy targeting CD19 showcased a 75% tumor-free survival rate in mouse models of B-cell lymphoma. It bypasses the need for lymphodepleting chemotherapy while reducing the risk of infection.
- Multiple Myeloma: Studies exploring the targeting of BCMA through in vivo methods using clinical data are still emerging.
Autoimmune Diseases
- Systemic Lupus Erythematosus (SLE): In vivo CAR-T cells targeting B-cell surface antigens showcased promise in depleting pathogenic B cells in primate models. It resulted in predominantly naive, reconstituted B cells, reflecting an immune system reset conducive to long-term remission.
- Other Autoimmune Conditions: Further studies and research are expanding to health complications such as rheumatoid arthritis and multiple sclerosis, where B-cell depletion could help modulate autoimmune pathology.
Solid Tumors
- Addressing Challenges:The primary challenges posed by solid tumors stem from immunosuppressive microenvironments and antigen heterogeneity. In vivo CAR-T therapy, on the other hand, helps refine this situation with armored CARs that are specifically designed to secrete cytokines or express checkpoint inhibitors, thereby improving T-cell persistence and infiltration capabilities.
- Early Progress: Preclinical models demonstrate encouraging results where in vivo-generated CAR-T cells infiltrate tumors and control growth after multiple doses.
Technological Advancements Shaping Its Future
Gene Delivery Platforms
- Viral Vectors: Retroviral and lentiviral vectors are currently being engineered to enhance the specificity of T cells. The surface modifications promise selective delivery and activation.
- Non-Viral Approaches: Lipid nanoparticles, inspired by mRNA vaccine technology, are being developed to encapsulate nucleic acids encoding CAR constructs, offering a more secure and scalable alternative.
In-Situ Immune Cell Engineering
- Direct Reprogramming: In vivo approaches supersede the need for ex vivo cell manipulation, reinfusion, and expansion, saving time, cost, and complexity.
- Multi-Cell Targeting: Currently, in vivo engineering prospects are being explored for other immune cells, including macrophages and NK cells, to enhance their efficacy in solid tumors.
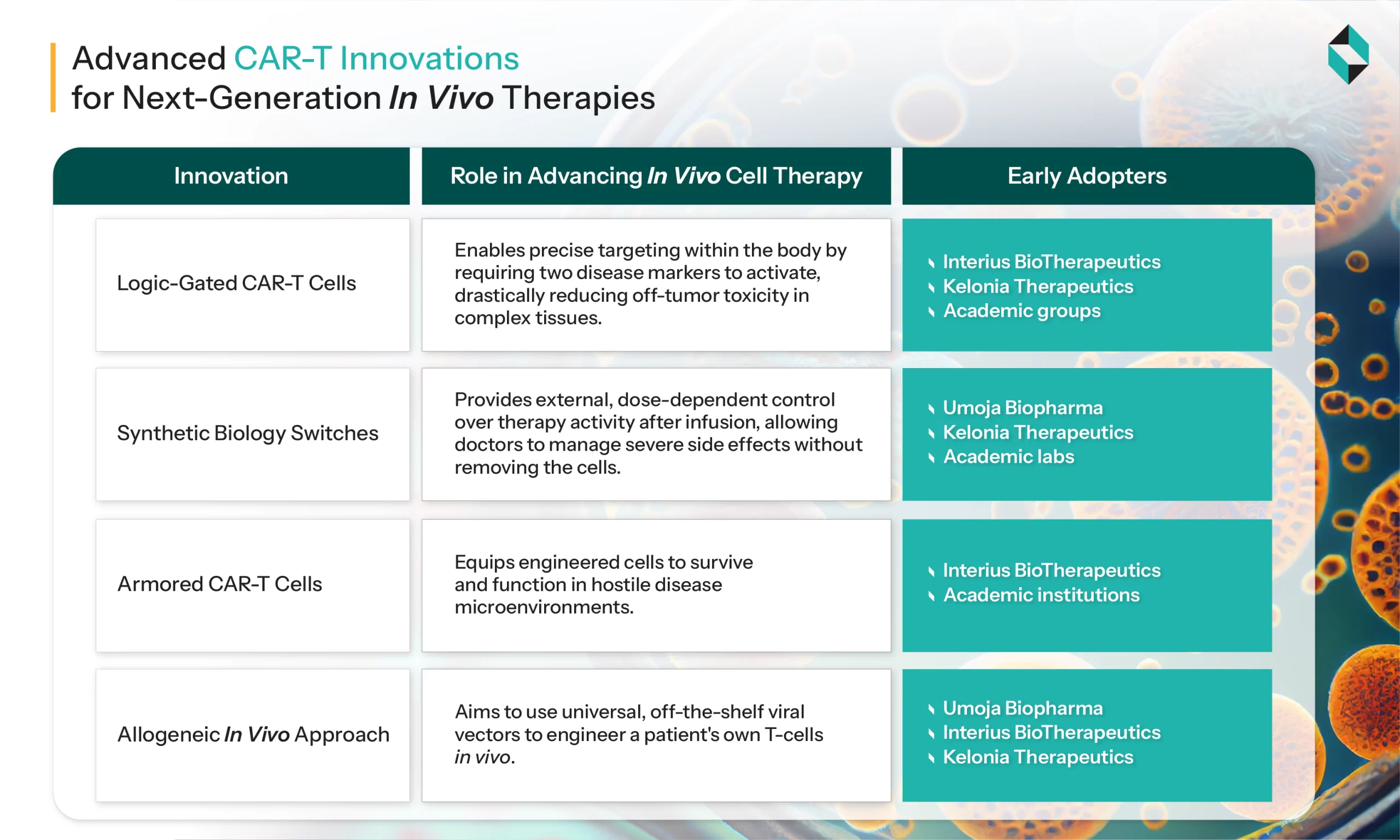
Next-Generation CAR Designs
- Modular CAR Constructs: Recent advancements in CAR structure, including the use of nanobodies, transmembrane domains, and optimized hinges, are enhancing specificity, persistence, and function.
- Self-Activating and Proliferative Signals: The incorporation of proprietary signaling domains enables the robust proliferation and persistence of CAR-T cells in vivo, even without preparative lymphodepletion.
Expanding Indications
- Solid Tumors: In vivo CAR-T approaches are being moulded for solid tumors treatment. While traditional CAR-T cell therapy struggled due to poor infiltration and immunosuppressive microenvironments, in vivo cell therapy promises better results.
- Non-Oncologic Diseases: There is growing interest in applying in vivo CAR-T for autoimmune diseases and other non-cancer indications.
Specific Challenges and Limitations of In-Vivo CAR-T Cell Therapy
- Antigen Selection and Heterogeneity: Most solid tumors are deficient in unique antigens, which raises the risk of off-tumor toxicity. Tumor antigen heterogeneity often results in antigen escape, as a result of which tumor cells lacking the targeted antigen survive.
- Tumor Microenvironment (TME): The immunosuppressive TME, including suppressive immune cells and inhibitory cytokines, impedes CAR-T cell function and persistence.
- Trafficking and Infiltration: CAR-T cells must migrate from the bloodstream into the tumor mass, a process hindered by the tumor stroma and the absence of specific chemokine signals.
- Persistence and Expansion: In-vivo generated CAR-T cells may have limited expansion and persistence due to the TME and lack of co-stimulatory signals.
- Safety and Off-Target Effects: Targeting tumor-associated antigens (TAAs) present on normal tissues often results in severe toxicities.
Key Biotech Startups Driving Innovation in In-Vivo CAR-T Cell Therapy Development
- Umoja Biopharma: It has developed VIVOVEC: IN VIVO GENE DELIVERY PLATFORM. It features a third-generation lentiviral vector technology to deliver genes that help the body make its own CAR-T cells.
- Capstan Therapeutics: Its goal is to utilize messenger RNA technology to coax the body into making its own CAR-T cells
- Interius BioTherapeutics: Interius BioTherapeutics is an early-stage biotech focused on treating hematologic malignancies by generating CAR-T cells directly in vivo (INT2104).
- Ensoma: Ensoma is carving out the viral genome of an adenovirus and using it to carry gene editing instructions into the body, developing one-time in vivo treatments.
The Future of In-Vivo CAR-T Cell Therapy
As of April 2024, 1,580 CAR-T clinical trials were registered globally, with the majority focusing on ex vivo approaches. In comparison to them, in vivo is an emerging field that will undoubtedly transform the future of cancer treatment. However, it is rapidly growing, expanding with technological and regulatory advancements, with the FDA, EMA, and other regulatory bodies stepping in. In the near future, further innovation and research efforts will lead to the optimization of in vivo CAR-T for non-cancer diseases, enhance the selectivity of gene delivery vehicles, develop robust safety controls, and modify genetic payloads for personalized therapy.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.