Advancements in Healthcare – Neurostimulation Devices
Neurostimulation devices are revolutionary in treating various neurological disorders by modulating the nervous system’s activity through electrical impulses. The market introduction involves rigorous clinical trials, regulatory approvals, and education of healthcare professionals and patients about their benefits. This process requires collaboration between manufacturers, clinicians, and regulatory authorities to ensure safety, efficacy, and widespread adoption.
Neurostimulation devices vary in their application, invasiveness, and mechanism of action, but they all have the common application of modulating neural activity to alleviate symptoms and improve quality of life.
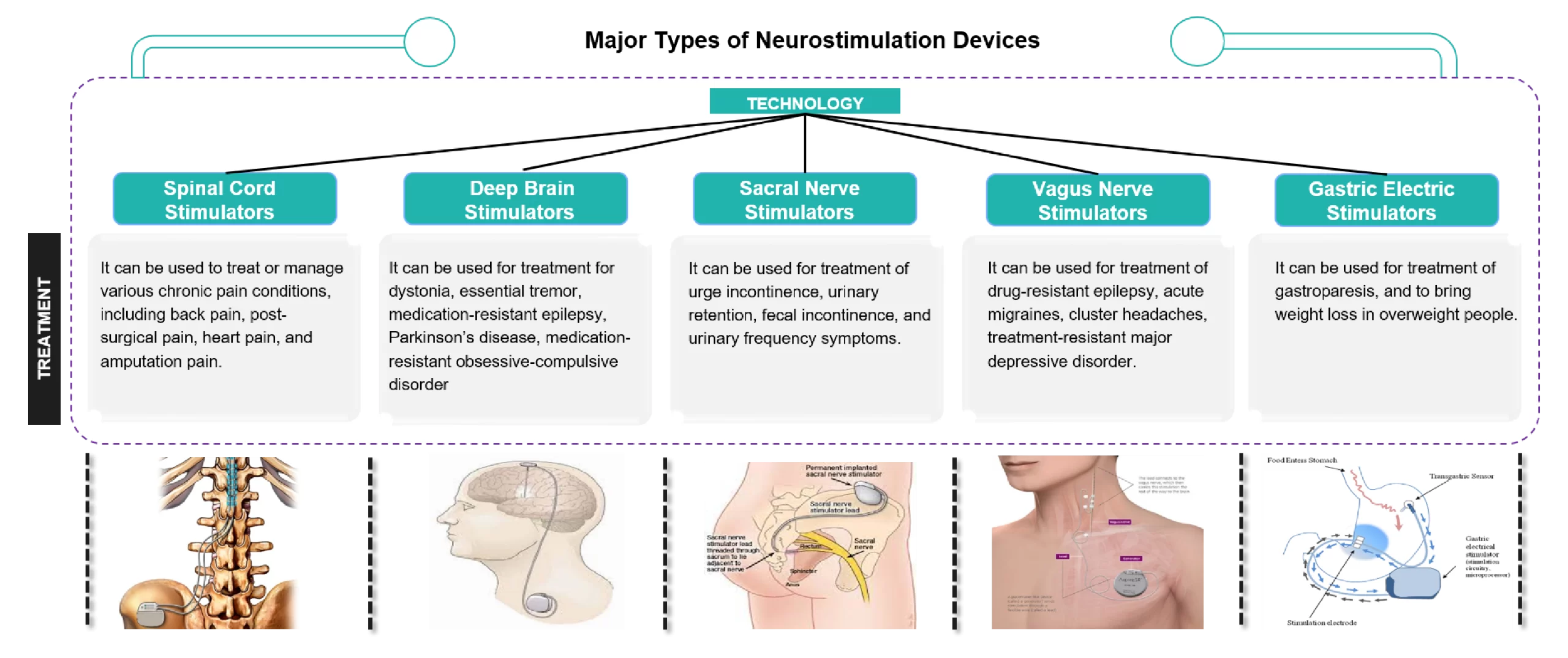
Figure 1: Different Types of Neurostimulation Devices
- Biomaterials & Bioelectronics
The technology is used to provide self-powered neurostimulation to achieve closed-loop therapeutic solutions and personalized neurostimulation.
- Wirelessly Powered Closed-Loop Neuro-modulation
The assembly uses implantable and wearable accessories to self-power the device to achieve wireless power transfer technology.
- Wireless Powered Implantable Closed Loop Neuro-stimulation
The implantable system is externally powered by NFC and a proximity-integrated circuit card to power the implantable device externally.
- Neuro-stimulation Electrodes
The neurostimulation device is equipped with flexible, low-cost, and biocompatible electrodes made from silicone polymer and nano clay, providing economic neural sensing applications.
The overview of neurostimulation devices available in the market, each offering innovative solutions for managing neurological conditions and improving patients’ quality of life.
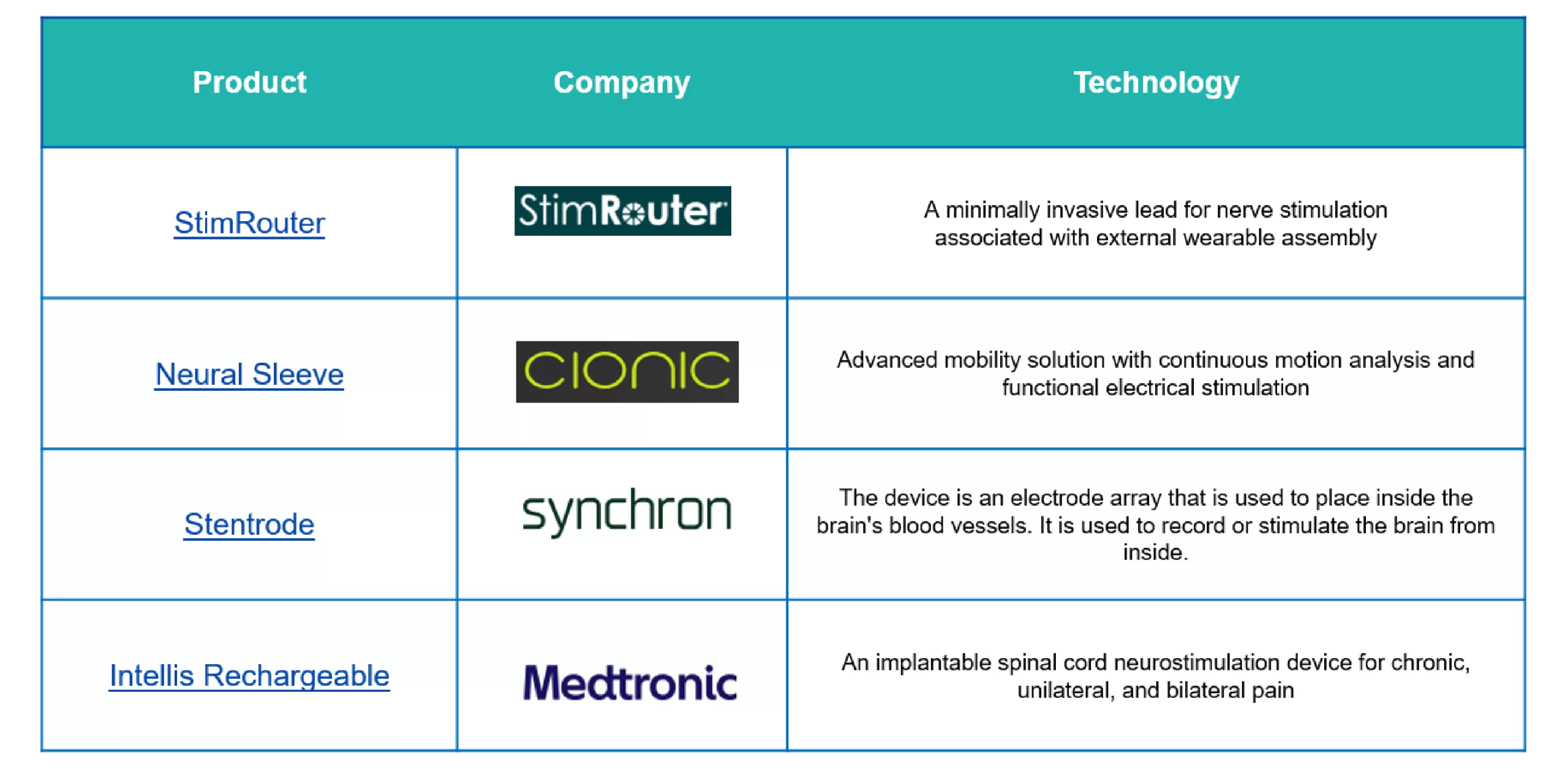
Figure 2: Product Overview
Neurostimulation devices’ past, present, and future demonstrate a remarkable evolution in technology, application, and potential impacts.
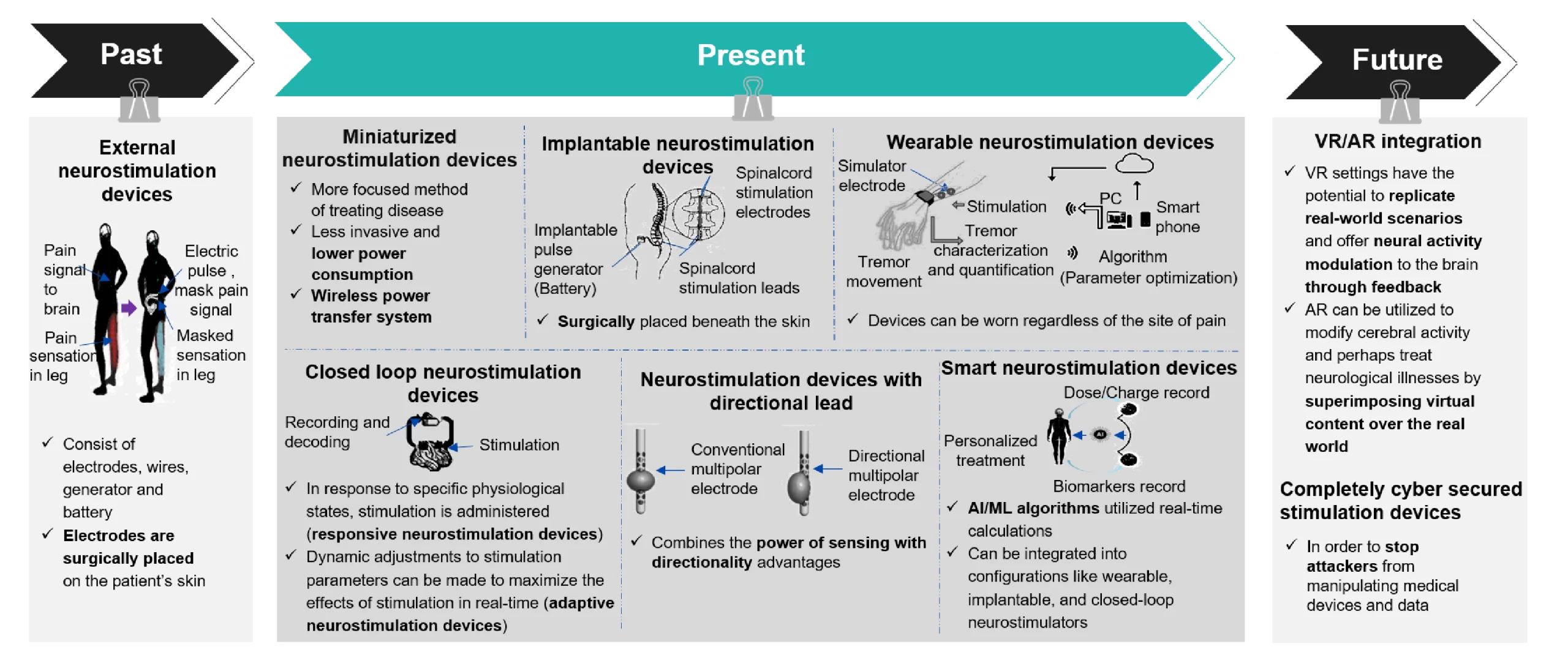
Figure 3: Evolution in Neurostimulation Devices
Regulatory approval is crucial for neurostimulation devices to examine their safety, efficacy, quality assurance, and patient protection to facilitate their adoption and use in brain and spinal clinical practice.
- Rechargeable Deep Brain Stimulation: Abbott received FDA approval for Liberta RC, a rechargeable deep brain stimulation system, on 25 January 2024. The technology is the world’s smallest and has the longest charge of any DBS technology on the market.
- Spinal Cord & Peripheral Nerve Stimulation: On the 10th of June 2022, Nalu Medical Inc. received FDA approval for the Nalu Neurostimulation System, which can be used for spinal cord stimulation & peripheral nerve stimulation.
- Percept™ RC Neurostimulator: In January 2024, Medtronic received approval from the FDA for the Percept RC deep brain stimulation system, used to treat Parkinson’s disease, tremors, dystonia, and epilepsy.
- Wavewriter Spinal Cord Stimulator (SCS) Systems: On 7th February 2024, Boston Scientific received approval from the FDA for wavewriter SCS systems to treat chronic low back and leg pain in people without prior back surgery.
- Micro-implant Neurostimulation Therapy System: In April 2023, Neuspera Medical obtained FDA approval for a micro-implant neurostimulation therapy system. The system deploys a wearable device and a wireless platform for peripheral nerve stimulation.
Startups in neurostimulation devices are a blend of innovation, regulatory compliance, and clinical validation. The focus of these startups is on developing safe, effective, and scalable technologies. These companies collaborate with clinicians, researchers, and investors to advance technology and market growth.
Nerivio
Nerivio is a drug-free, remote electrical neuromodulation wearable system for migraine treatment and prevention. It stimulates the upper arm nerves, which carry pain signals to the brain. The device is controlled by a smartphone app and worn on the upper arm for 45 minutes daily to prevent migraine pain without any medications.
The Stentrode
Synchron developed the stentrode, an endovascular electrode array. The device is designed to record or stimulate the brain from inside the blood vessels.
Auricular VNS
Vagustim is the world’s first mobile app-controlled, noninvasive bilateral auricular Vagus nerve stimulation service. The device provides gentle electrical stimuli through the ears to stimulate the Vagus nerve noninvasively. It is integrated with Bluetooth hardware, controlled by a mobile application, and used to collect data for customized therapy.
Neurostimulation devices have made immense advancements in treating various neurological and psychiatric disorders. However, their development and use face significant challenges, which are disclosed along with their solutions.
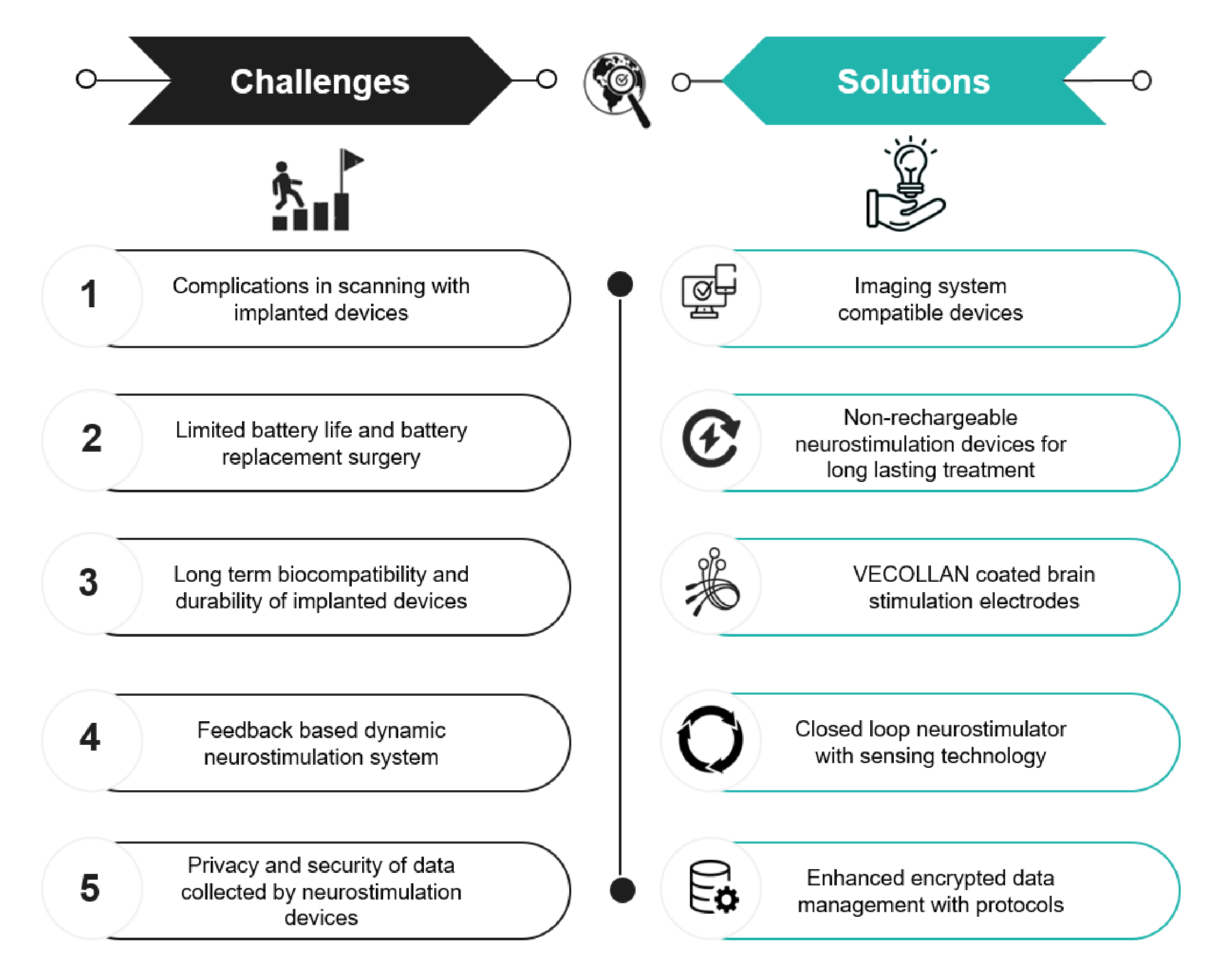
Figure 4: Top Challenges and Solutions
The key market highlights are exciting technology development through mergers and acquisitions to advance neuro-stimulation applications.
- 8th January 2024: Boston Scientific announced the acquisition of Axonics, which is a maker of neurostimulation devices used to treat urinary and bowel dysfunction
- 5th September 2023: Amber Therapeutics acquired Bioinduction Limited to enhance its integrated development and manufacturing portfolio of closed-loop neuromodulation therapies
- September 2023: A collaborative study involving the University of Surrey, the University of Oxford, Radboud University, and Loughborough University was conducted to explore the scope of neurostimulation to boost mathematical learning. For this, high-frequency transcranial random noise stimulation was applied to stimulate certain brain regions and enhance the mathematical capabilities of participants. The research suggests a positive impact of brain stimulation on mathematical skills.
- 28th April 2022: Inspire Medical Systems invested in Ensodata and Ognomy. The companies create software and diagnostic devices for neurostimulation systems
- 14th June 2021: KT & NeuroSigma announced a strategic digital health partnership to develop and commercialize neuroelectronic therapies inside and outside Korea
Over the last few years, there has been a rise in competitiveness within the neurostimulation devices sector. To remain competitive, businesses will need to broaden the range of services they provide and look into new revenue streams. The growing demand for rapid and accurate stimulation is due to the increasing prevalence of neural diseases and the need for early treatment. Market competitors in neurostimulation devices with their categorization are represented below:

Figure 5: Major Institutes and Key Players
The neuro-stimulation device market is drastically growing in the healthcare domain. It provides novel solutions for a wide range of neurological conditions. These solutions stimulate the neural network through electrical or magnetic stimulation to normalize function and symptoms. The technology eases the suffering of patients who are suffering from depression, chronic pain, and addiction to improve their quality of life.
The adoption and development of neurostimulation devices face various challenges. These include ensuring safety, efficacy, regulatory approvals, optimized device design, and application to address issues related to market coverage.
The neurostimulation device market is witnessing technological advancements and increased adoption by healthcare providers and patients. Moreover, it’s observing enhanced investments from established startups and companies.
Let's Take the Conversation Forward
Reach out to Stellarix experts for tailored solutions to streamline your operations and achieve
measurable business excellence.